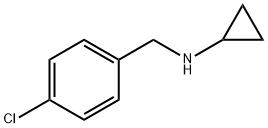
N-(4-CHLOROBENZYL)-N-CYCLOPROPYLAMINE synthesis
- Product Name:N-(4-CHLOROBENZYL)-N-CYCLOPROPYLAMINE
- CAS Number:19271-24-0
- Molecular formula:C10H12ClN
- Molecular Weight:181.66

623-03-0
444 suppliers
$6.00/25g
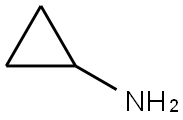
765-30-0
486 suppliers
$10.00/25g
![Benzenemethanamine, 4-chloro-N-[(4-chlorophenyl)methyl]-](/CAS/20210305/GIF/21913-13-3.gif)
21913-13-3
0 suppliers
inquiry

19271-24-0
22 suppliers
$65.00/100mg
Yield:-
Reaction Conditions:
with C22H27ClN3Ru(1+)*F6P(1-);hydrogen in isopropyl alcohol at 80; under 45004.5 Torr; for 12 h;
Steps:
4.6 General Procedure for the catalytic hydrogenation of nitriles
General procedure: All of the hydrogenation reactions were performed at constant pressures using a stainless steel 50mL Parr hydrogenation reactor. The reactor was flushed three times with hydrogen gas at 2-4bar prior to the addition of catalyst and substrate. Catalyst 1 (0.02mmol), nitrile (1mmol), and dodecane (1mmol) in case of symmetrical amine synthesis or, catalyst 1 (0.02mmol), nitrile (1mmol), amine (3mmol) and dodecane (1mmol) in case of asymmetrical amine synthesis were dissolved in iPrOH (5mL) under a nitrogen atmosphere. The solution was then injected into the reactor against a flow of hydrogen gas. The hydrogen gas was adjusted to 60bar. The temperature of the system was maintained at 80°C using a thermostat. Small aliquots of the reaction mixture were withdrawn after 12h with a syringe and diluted with 2mL of EtOAc and passed through a very short column of silica and subjected to GC-MS analysis. A few selected secondary amines were purified by flash chromatography and characterized by 1H and 13C NMR spectra.
References:
Saha, Sayantani;Kaur, Mandeep;Singh, Kuldeep;Bera, Jitendra K. [Journal of Organometallic Chemistry,2016,vol. 812,p. 87 - 94]
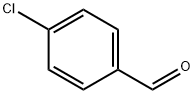
104-88-1
611 suppliers
$5.00/25g

765-30-0
486 suppliers
$10.00/25g

19271-24-0
22 suppliers
$65.00/100mg

104-88-1
611 suppliers
$5.00/25g

19271-24-0
22 suppliers
$65.00/100mg