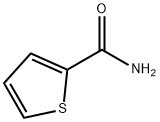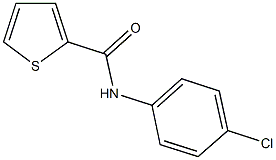
N-(4-chlorophenyl)-2-thiophenecarboxamide synthesis
- Product Name:N-(4-chlorophenyl)-2-thiophenecarboxamide
- CAS Number:15950-35-3
- Molecular formula:C11H8ClNOS
- Molecular Weight:237.7053
Yield:15950-35-3 80%
Reaction Conditions:
with potassium carbonate;N,N`-dimethylethylenediamine in toluene at 110; for 48 h;Schlenk technique;Inert atmosphere;
Steps:
N-Arylated Benzamides 3
General procedure: An oven-dried Schlenk tube was charged with benzamide 1 (0.5 mmol), K2CO3 (207 mg, 1.5 mmol) and aryl iodide 2 (1.0 mol). The tube was evacuated and backfilled with N2 (3 ×), and then DMEDA (0.2 mmol) and anhyd toluene (5.0 mL) were added. The reaction mixture was stirred at 110 ° C for 48 h. H2O was added and the crude product was extracted with EtOAc. The combined organic phases were washed with brine and H2O, dried (Na2SO4), and concentrated under reduced pressure. The product was purified by silica gel chromatographyto give the desired N-arylated benzamides (Table 2).
References:
Huang, Fei;Wu, San;Hu, Weiye;Zhang, Songlin [Synthesis,2018,vol. 50,# 5,p. 1090 - 1096] Location in patent:supporting information
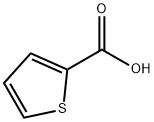
527-72-0
482 suppliers
$5.00/10g

15950-35-3
4 suppliers
inquiry