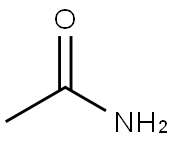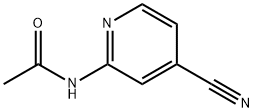
N-(4-CYANO-2-PYRIDINYL)-ACETAMIDE synthesis
- Product Name:N-(4-CYANO-2-PYRIDINYL)-ACETAMIDE
- CAS Number:939997-68-9
- Molecular formula:C8H7N3O
- Molecular Weight:161.16
Yield:939997-68-9 54%
Reaction Conditions:
with potassium phosphate;tris-(dibenzylideneacetone)dipalladium(0);4,5-bis(diphenylphosphino)-9,9-dimethylxanthene in 1,4-dioxane at 150; for 1 h;Microwave irradiation;
Steps:
1
A mixture of 2-chloroisonicotinonitrile (1.50 g, 10.8 mmol), acetamide (1.28 g, 21.7 mmol), tris(dibenzylideneacetone)dipalladium(0) (198 mg, 0.22 mmol), 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene (376 mg, 0.65 mmol), and tripotassium phosphate (2.76 g, 13.0 mmol) in dioxane (15 mL) is stirred at 150 oC for 1 hour under microwave irradiation. The resulting mixture is filtered through a pad of Celite, and the filtrate is concentrated in vacuo. The residue is purified by column chromatography on silica gel eluting with hexane / EtOAc (2:1 to 1:2) to give 950 mg (54% yield) of the title compound as a pale yellow solid. 1H-NMR (300 MHz, CDCl3) delta 8.52 (1H, s), 8.42 (1H, d, J = 5.1 Hz), 8.03 (1H, br s), 7.25 (1H, d, J = 5.1 Hz), 2.25 (3H, s), MS (ESI) m/z: 162 (M+H)+.
References:
WO2013/161308,2013,A1 Location in patent:Paragraph 0177
42182-27-4
245 suppliers
$10.00/1g

75-36-5
562 suppliers
$17.92/100G

939997-68-9
7 suppliers
inquiry