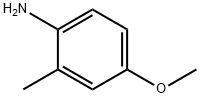
N-(4-methoxy-2-methyl-phenyl)benzamide synthesis
- Product Name:N-(4-methoxy-2-methyl-phenyl)benzamide
- CAS Number:61495-08-7
- Molecular formula:C15H15NO2
- Molecular Weight:241.29
Yield:61495-08-7 42%
Reaction Conditions:
with triethylamine in dichloromethane at 20; for 16 h;
Steps:
N-(4-Methoxy-2-methylphenyl)benzamide (S-9)
Benzoylchloride (3.16 mL, 27.3 mmol) was added dropwise to a CH2Cl2 (50 mL) solution of 5-methoxy-2-methylaniline (S-8) (2.50 g, 18.2 mmol) and Et3N (5.10 mL, 36.4 mmol). The solution was stirred at room temperature (16 h). An aqueous saturated NaHCO3 solution (50 mL) was added and the layers separated. The aqueous layer was washed with CH2Cl2 (2 x50 mL). The organic layers were combined washed with an aqueous HCl solution (2N, 50 mL) and concentrated under vacuum. The residue was recrystallized in toluene to obtain S-9 as a grey solid (1.80 g, 42%): Rf = 0.29 (heptane/EtOAc 8/2); 1H NMR (CDCl3) d 2.32 (s, CH3), 3.83 (s, OCH3), 6.80-6.82 (m, 2 ArH), 7.49-7.60 (m, 1ArH, NH, 3 PhH), 7.90 (d, J = 7.6 Hz, 2 PhH); 13C NMR (CDCl3) d 18.2 (CH3),55.4 (OCH3), 111.7, 116.1 (C5, C6), 125.7, 127.1 (2 CH), 128.6 (Cquater), 128.8, 131.7 (2 CH), 132.6, 135.0 (2 Cquater), 157.5 (C(O)).
References:
Salomé, Christophe;Ribeiro, Nigel;Chavagnan, Thierry;Thuaud, Frédéric;Serova, Maria;De Gramont, Armand;Faivre, Sandrine;Raymond, Eric;Désaubry, Laurent [European Journal of Medicinal Chemistry,2014,vol. 81,p. 181 - 191] Location in patent:supporting information
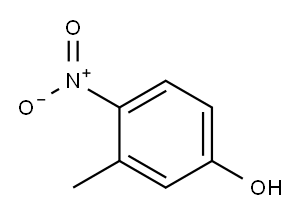
2581-34-2
260 suppliers
$10.00/5G

61495-08-7
2 suppliers
inquiry
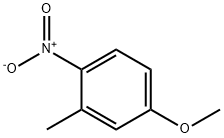
5367-32-8
180 suppliers
$10.00/5g

61495-08-7
2 suppliers
inquiry