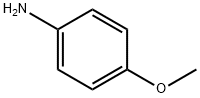
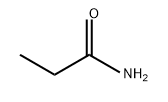
N-(4-METHOXYPHENYL)PROPIONAMIDE synthesis
- Product Name:N-(4-METHOXYPHENYL)PROPIONAMIDE
- CAS Number:2760-31-8
- Molecular formula:C10H13NO2
- Molecular Weight:179.22
Yield:2760-31-8 93%
Reaction Conditions:
Stage #1: propionic acidwith diatomite earth(at)IL/ZrCl4 (Lewis acidic ionic liquid immobilized on diatomite earth) in toluene; for 0.0833333 h;Green chemistry;
Stage #2: 4-methoxy-aniline in toluene at 20; for 0.333333 h;Sonication;Green chemistry;
Steps:
General procedure for the preparation of benzamides
General procedure: Benzoic acid derivatives (1 mmol) and heterogeneous catalyst (10 mg) were mixed for 5 min in 1 mL of anhydrous toluene. Then, the amine (1.2 mmol) was added and the mixture was reacted under ultrasound for about 15-60 min at room temperature. After completion of the reaction (monitored by TLC), the catalyst was separated through filtration and the solvent was removed in vacuo. The obtained residue was dissolved in chloroform (10 ml) and washed with 10% NaHCO3 (10 ml) and HCl (1 M, 10 ml). The organic layer was extracted and dried over Na2SO4 and concentrated under reduced pressure to afford the amide, which was purified by recrystallization or column chromatography.
References:
Ahmadi, Masoumeh;Moradi, Leila;Sadeghzadeh, Masoud [Research on Chemical Intermediates,2018,vol. 44,# 12,p. 7873 - 7889]
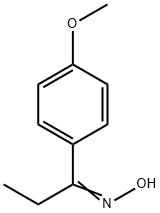
24316-59-4
0 suppliers
inquiry

2760-31-8
22 suppliers
$45.00/50mg