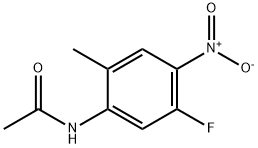
N-(5-Fluoro-2-methyl-4-nitrophenyl)acetamide synthesis
- Product Name:N-(5-Fluoro-2-methyl-4-nitrophenyl)acetamide
- CAS Number:633327-49-8
- Molecular formula:C9H9FN2O3
- Molecular Weight:212.18
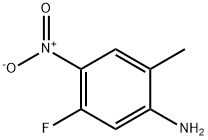
633327-50-1
79 suppliers
$35.00/5g

108-24-7
5 suppliers
$14.00/250ML

633327-49-8
21 suppliers
$60.00/100mg
Yield:633327-49-8 89%
Reaction Conditions:
at 15; for 36 h;Inert atmosphere;
Steps:
23 Preparation 23: /v-(5-Fluoro-2-methyl-4-nitrophenyl)acetamide
5-fluoro-2-methyl-4-nitroaniline (9.5 g, 56 mmol) was added in portions to AC2O (100 mL) at 15 °C. The reaction mixture was stirred at about 15 °C for about 36 h. The solids were collected by filtration and rinsed with water (3 x 50 mL). The solids were dried to provide the title compound. Yield: 6.3 g. The filtrate was extracted with EtOAc (100 mL). The organic layer was washed with water (2 x 100 mL), sat. aq. Na2HCOs (3 x 100 mL), dried (Na2SC>4), filtered and concentrated. The crude product was purified by chromatography to provide additional title compound. Yield: 4 g. Both batches of MR (400
References:
WO2022/130171,2022,A1 Location in patent:Page/Page column 57
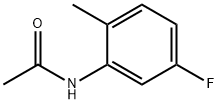
366-49-4
66 suppliers
$55.00/250mg

633327-49-8
21 suppliers
$60.00/100mg
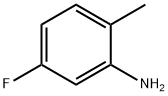
367-29-3
272 suppliers
$7.00/5g

633327-49-8
21 suppliers
$60.00/100mg