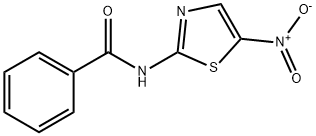
N-(5-nitro-2-thiazolyl)benzamide synthesis
- Product Name:N-(5-nitro-2-thiazolyl)benzamide
- CAS Number:64398-84-1
- Molecular formula:C10H7N3O3S
- Molecular Weight:249.25
Yield:64398-84-1 87%
Reaction Conditions:
with triethylamine in dichloromethane at 20;
Steps:
2 General method of synthesis of amides (9-12)
General procedure: To a solution of 2-amino-5-nitrothiazole (0.3 g, 0.0020 mol) in dichloromethane was added 1.2 molar equiv of triethylamine (TEA). After the reaction mixture was stirred at 5 °C for 15 min, acetic anhydride (0.0100 mol, 5 equiv), or respectively acyl chlorides (0.0022 mol, 1.1 equiv) were added drop-wise. The reaction mixture was stirred at room temperature for 4-24 h. After complete conversion, the solvent was removed in vacuo and the residue was neutralized with saturated NaHCO3 solution. The precipitated solids were recrystallized from a mixture of solvents.
References:
Nava-Zuazo, Carlos;Chávez-Silva, Fabiola;Moo-Puc, Rosa;Chan-Bacab, Manuel Jesús;Ortega-Morales, Benjamín Otto;Moreno-Díaz, Hermenegilda;Díaz-Couti?o, Daniel;Hernández-Nú?ez, Emanuel;Navarrete-Vázquez, Gabriel [Bioorganic and Medicinal Chemistry,2014,vol. 22,# 5,p. 1626 - 1633]
65-85-0
1333 suppliers
$10.00/25g

64398-84-1
12 suppliers
$87.00/1g

