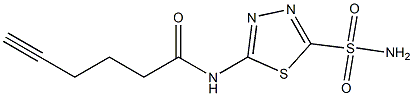
N-(5-sulfamoyl-1,3,4-thiadiazol-2-yl)hex-5-ynamide synthesis
- Product Name:N-(5-sulfamoyl-1,3,4-thiadiazol-2-yl)hex-5-ynamide
- CAS Number:1623789-42-3
- Molecular formula:C8H10N4O3S2
- Molecular Weight:274.32
14949-00-9
79 suppliers
$60.00/100mg

55183-45-4
23 suppliers
$279.23/1gm:

1623789-42-3
5 suppliers
inquiry
Yield:1623789-42-3 55%
Reaction Conditions:
with N,N-dimethyl-formamide at 0 - 20; for 48 h;Inert atmosphere;
Steps:
N-(5-sulfamoyl-1,3,4-thiadiazol-2-yl)hex-5-ynamide (13).
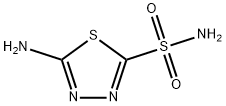
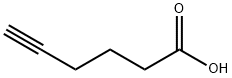
The 5-hexynoic acid (100 mg, 0.89 mmol,3.1 equiv.) was treated with an excess of thionyl chloride (2 mL, 27.56 mmol) at 0 C, and thereaction mixture was heated to 70 C and stirred for 1 h. 5-Hexynoyl chloride was obtained byevaporation of the excess of thionyl chloride and dried under vacuum. A solution of 5-amino-1,3,4-thiadiazole-2-sulfonamide (50 mg, 0.28 mmol, 1.0 equiv.) in anhydrous DMF (2 mL) was slowly addedinto 5-hexynoyl chloride at 0 C under N2. The reaction mixture was warmed to rt and stirred for 48 h.The reaction mixture was then concentrated and purified by flash chromatography (with a gradient ofEtOAc/Hexanes = 1:4 to 1:1 to 100% EtOAc; silica gel) to give the product as an off-white solid (42 mg,55%). 1H NMR (400 MHz, DMSO-d6): d 13.02 (s, 1H), 8.31 (s, 2H), 2.82 (t, 1H, J = 2.4 Hz), 2.63 (t, 2H,J = 7.2 Hz), 2.21-2.25 (m, 2H), 1.78-1.81 (m, 2H). ESI-MS: m/z 297.1 [M + Na]+.
References:
Chen, Kuo-Ting;Nguyen, Kevin;Ieritano, Christian;Gao, Feng;Seimbille, Yann [Molecules,2019,vol. 24,# 1,art. no. 23]
53293-00-8
192 suppliers
$10.00/250mg

1623789-42-3
5 suppliers
inquiry