
N-(6-Chloropyridazin-3-yl)pivalamide synthesis
- Product Name:N-(6-Chloropyridazin-3-yl)pivalamide
- CAS Number:147362-88-7
- Molecular formula:C9H12ClN3O
- Molecular Weight:213.66
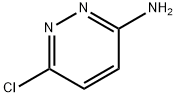
5469-69-2
374 suppliers
$6.00/10g
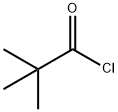
3282-30-2
367 suppliers
$22.00/100ml

147362-88-7
36 suppliers
inquiry
Yield:147362-88-7 62 %
Reaction Conditions:
with triethylamine in tetrahydrofuran at 0 - 20;
Steps:
N- ( 6 -ch loropy r idazin-3-y I) -2,2-di methyl- propanam ide
TEA (1 .68 L, 1 .16 mol) was added to a solution of 6-chloropyridazin-3-amine (500 g, 3.87 mol) in THF (2.5 L) at 0°C. Pivaloyl chloride (945 mL, 7.72 mol) was added dropwise over 2 hours at the same temperature and stirred for 16 hours at room temperature before it was quenched with water (1 .5 L) and extracted with EtOAc (2 x 3.0 L) . The combined organic phases were washed with water (2.0 L) , brine (1 .5 L) , dried over Na2SC>4, filtered and concentrated in vacuo. The crude solid was stirred with DCM (1 .5 L) for 30 m in, filtered, washed with Et20 (1 .5 L) and concentrated in vacuo to afford the title compound as an off white solid (510 g, 62% yield) . 1H NMR (400 MHz, CDCI3) ? 8.52 (d, J= 9.37 Hz, 2H) , 7.50 (dd, J= 9.37, 0.65 Hz, 1 H) , 1 .35 (s, 9H) ; LCMS (Method 4, ACQUITY BEH C18 column, 0.05% FA in water with MeCN) (ES) : m/z 214 [ M+ H] + , RT = 1 .60 min.
References:
WO2023/25783,2023,A1 Location in patent:Page/Page column 32