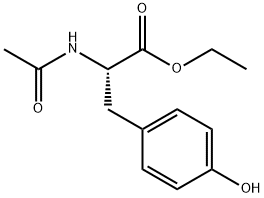
N-Acetyl-3,5-diiodo-L-tyrosine ethyl ester synthesis
- Product Name:N-Acetyl-3,5-diiodo-L-tyrosine ethyl ester
- CAS Number:21959-36-4
- Molecular formula:C13H15I2NO4
- Molecular Weight:503.07

64-17-5
746 suppliers
$10.00/50g
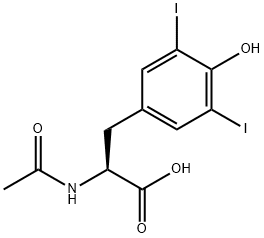
1027-28-7
168 suppliers
$12.00/5g

21959-36-4
129 suppliers
$110.00/5g
Yield: 88.3%
Reaction Conditions:
Stage #1:ethanol;N-acetyl-3,5-diiodo-L-tyrosine at 20; for 0.5 h;
Stage #2: with thionyl chloride at 20 - 50; for 1 h;
Steps:
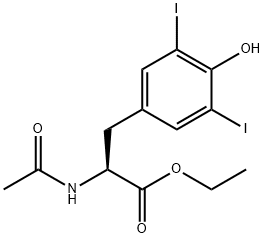
3; 4 Example 3 Synthesis of N-acetyl-3,5-diiodo-L-tyrosine ethyl ester LT-2
60 g of N-acetyl-3,5-diiodo-L-tyrosine and 900 mL of absolute ethanol were added to a 2 L reaction flask, and stirred at room temperature for 30 minutes, then 5.8 mL of thionyl chloride was added, and then the temperature was raised to 50 °C.The reaction was stirred for 1 hour, and after the reaction was completed, the mixture was concentrated to dryness under reduced pressure. The crude product was dissolved in 500 mL of dichloromethane and then washed twice with 300 mL of saturated sodium hydrogen carbonate solution.The crude product is filtered off with suction and then beating in 100mL dichloromethane and concentrated to dryness The organic phase was dried over anhydrous sodium sulfate and dried under reduced pressure,The mixture was washed with 10 mL of dichloromethane, and the obtained cake was dried under vacuum at 40 ° C for 5 hours to obtain 56.1 g of a white powdery solid. The yield was 88.3% and the purity was 98.52%
References:
Famahe (Tianjin) Technology Co., Ltd.;Jie You;Qian Yu CN109761830, 2019, A Location in patent:Paragraph 0009; 0030; 0031
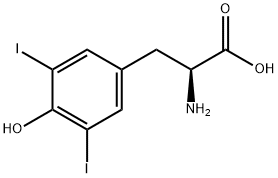
300-39-0
271 suppliers
$10.00/5g

21959-36-4
129 suppliers
$110.00/5g
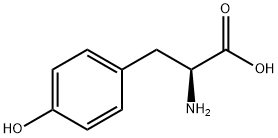
60-18-4
863 suppliers
$15.00/25g

21959-36-4
129 suppliers
$110.00/5g