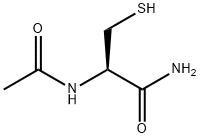
N-Acetylcysteine amide synthesis
- Product Name:N-Acetylcysteine amide
- CAS Number:38520-57-9
- Molecular formula:C5H10N2O2S
- Molecular Weight:162.21

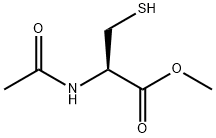
7652-46-2
74 suppliers
$40.00/250mg

38520-57-9
94 suppliers
$60.00/1mg
Yield:38520-57-9 93%
Reaction Conditions:
with ammonium hydroxide in water at 20; for 168 h;
Steps:
(R)-2-Acetylamino-3-merca to-propionamide (\2) (used during synthesis of 4)
(R)-2-Acetylamino-3-merca to-propionamide (2) (used during synthesis of 4) (R)-2-Acetylamino-3-mercapto-propionic acid methyl ester (177 mg, 1.0 mmol) was dissolved in NH4OH (28% aq., 5ml_) and the reaction mixture stirred at rt for 7 days. The solvent was evaporated in vacuo to give a white solid (150mg, 0.93 mmol, 93%).
References:
WO2015/181551,2015,A1 Location in patent:Page/Page column 35
16359-16-3
0 suppliers
inquiry

38520-57-9
94 suppliers
$60.00/1mg
59587-09-6
113 suppliers
$22.00/100mg

38520-57-9
94 suppliers
$60.00/1mg
616-91-1
907 suppliers
$5.00/5g

38520-57-9
94 suppliers
$60.00/1mg

