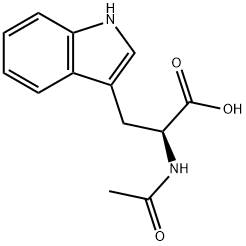
N-Acetyl-L-tryptophan synthesis
- Product Name:N-Acetyl-L-tryptophan
- CAS Number:1218-34-4
- Molecular formula:C13H14N2O3
- Molecular Weight:246.26

75-36-5
563 suppliers
$17.92/100G
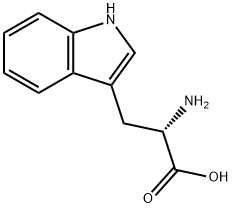
73-22-3
947 suppliers
$5.00/250mg

1218-34-4
372 suppliers
$10.00/1g
Yield:1218-34-4 80%
Reaction Conditions:
with sodium hydroxide in acetone; pH=> 10 at 20;
Steps:
4 4.1.8.4. Syntheses of N-acetamido-L-tryptophan (10b)
4.1.8.4
Syntheses of N-acetamido-l-tryptophan (10b)
l-Tryptophan (1.00 g, 4.95 mmol) was added to a solution of acetone (20 mL) and 2 N NaOH (15 mL).
Acetyl chloride (0.821 mL, 9.90 mmol) and 2 N NaOH were added simultaneously slowly at room temperature.
The solution was maintained at a pH greater than 10 and stirred for 1 h.
The acetone was evaporated in vacuo and the remaining solution was made acidic with 3 N HCl.
The aqueous layer was extracted with EtOAc (3 * 50 mL).
The combined organic layers were washed with brine and dried over anhydrous Na2SO4.
The solution was concentrated in vacuo to give N-acetamido-l-tryptophan 10b (0.96 g, 80%) as a colorless solid. ESI/MS m/z: 246.1 [M+H]+; 1H NMR (500 MHz, DMSO) δ 12.55 (s, 1H), 10.84 (s, 1H), 8.14 (d, J = 8.0 Hz, 1H), 7.52 (d, J = 8.0 Hz, 1H), 7.33 (d, J = 8.0 Hz, 1H), 7.13 (d, J = 2.0 Hz, 1H), 7.06 (dd, J = 11.0, 4.0 Hz, 1H), 6.98 (t, J = 7.5 Hz, 1H), 4.46 (td, J = 8.5, 5.0 Hz, 1H), 3.15 (dd, J = 14.5, 5.0 Hz, 1H), 2.98 (dd, J = 14.5, 8.5 Hz, 1H), 1.80 (s, 3H).
References:
Lan, Jin-Shuai;Xie, Sai-Sai;Li, Su-Yi;Pan, Long-Fei;Wang, Xiao-Bing;Kong, Ling-Yi [Bioorganic and Medicinal Chemistry,2014,vol. 22,# 21,p. 6089 - 6104]

108-24-7
5 suppliers
$14.00/250ML

73-22-3
947 suppliers
$5.00/250mg

1218-34-4
372 suppliers
$10.00/1g
507-09-5
300 suppliers
$12.00/5g

73-22-3
947 suppliers
$5.00/250mg

1218-34-4
372 suppliers
$10.00/1g

70082-70-1
1 suppliers
inquiry

1218-34-4
372 suppliers
$10.00/1g
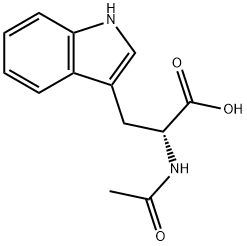
2280-01-5
171 suppliers
$6.00/250mg
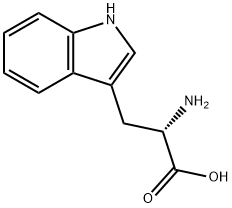
54-12-6
359 suppliers
$6.00/5g

1218-34-4
372 suppliers
$10.00/1g