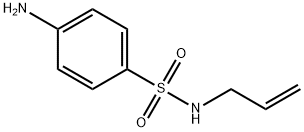
N-ALLYL-4-AMINO-BENZENESULFONAMIDE synthesis
- Product Name:N-ALLYL-4-AMINO-BENZENESULFONAMIDE
- CAS Number:117057-51-9
- Molecular formula:C9H12N2O2S
- Molecular Weight:212.27
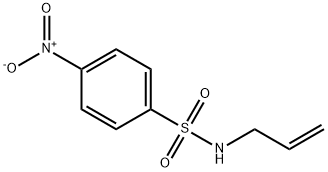
100704-44-7
2 suppliers
inquiry

117057-51-9
17 suppliers
$46.00/1g
Yield:117057-51-9 100%
Reaction Conditions:
with hydrogenchloride;tin in ethanol;water;Reflux;
Steps:
31 4.6.2. General procedure B (step 2)
General procedure: Concentrated HCl (10-15 ml) was added slowly to a mixture of 4-nitrobenzenesulfonamide or 4-nitrobenzamide analog (13a-q,1.4 mmol, 1 eq) and Sn granules (2-2.5 eq) in ethanol (10 mL). The reaction mixture was refluxed for 3.5-6 h. When the TLC plates howed that there is no more starting material left, the reaction mixture was cooled down to rt. NaOH (30% or 5M, aq) was added to the mixture until it became basic. The mixture was extracted with EA or DCM and the organic layer was dried with Na2SO4. The solvent was evaporated. The product was purified with column chromatography when required. 4.6.4.31
N-Allyl-4-aminobenzenesulfonamide (14n)
From 13n (0.29 g, 1.2 mmol) with general procedure B. Light orange solid (0.30 g, 100%) that was used without further purification. 1H NMR (DMSO-d6): δ 3.31 (2H, br s), 5.01 (1H, dq, J = 10.2, 1.6 Hz), 5.12 (1H, dq, J = 17.2, 1.7 Hz), 5.66 (1H, ddt, J = 17.2, 10.3, 5.6 Hz), 5.91 (2H, s), 6.60 (2H, d, J = 8.6 Hz), 7.27 (1H, s), 7.40 (2H, d, J = 8.6 Hz).
References:
Yrj?l?, Sari;Parkkari, Teija;Navia-Paldanius, Dina;Laitinen, Tuomo;Kaczor, Agnieszka A.;Kokkola, Tarja;Adusei-Mensah, Frank;Savinainen, Juha R.;Laitinen, Jarmo T.;Poso, Antti;Alexander, Amy;Penman, June;Stott, Lisa;Anskat, Marie;Irving, Andrew J.;Nevalainen, Tapio J. [European Journal of Medicinal Chemistry,2016,vol. 107,p. 119 - 132]
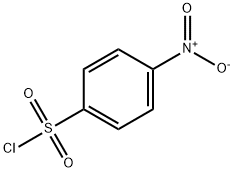
98-74-8
166 suppliers
$10.00/5g

117057-51-9
17 suppliers
$46.00/1g
![N-{4-[(allylamino)sulfonyl]phenyl}acetamide](/CAS/20180711/GIF/101568-44-9.gif)
101568-44-9
6 suppliers
inquiry

117057-51-9
17 suppliers
$46.00/1g

121-60-8
343 suppliers
$10.00/1g

117057-51-9
17 suppliers
$46.00/1g