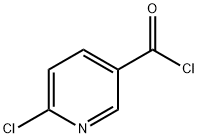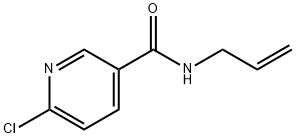
N-allyl-6-chloronicotinamide synthesis
- Product Name:N-allyl-6-chloronicotinamide
- CAS Number:915921-01-6
- Molecular formula:C9H9ClN2O
- Molecular Weight:196.63
69045-79-0
349 suppliers
$5.00/1g

107-11-9
1 suppliers
$29.09/25ml
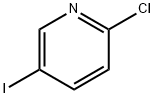
199620-15-0
0 suppliers
inquiry

915921-01-6
10 suppliers
inquiry
Yield:915921-01-6 76 %Chromat.
Reaction Conditions:
with palladium diacetate;1,8-diazabicyclo[5.4.0]undec-7-ene in 1,4-dioxane at 125; for 0.0833333 h;Microwave irradiation;
Steps:
4.2 Microwave-assisted palladium-catalysed aminocarbonylation
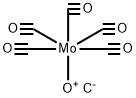
General procedure: The iodoaryl substrate (0.4mmol), allylamine (45μL, 0.6mmol), Pd(OAc)2 (2.2mg, 0.01mmol), Mo(CO)6 (105.6mg, 0.4mmol), DBU (180μL, 1.2mmol) were dissolved in dioxane (3mL) and placed into a microwave vial. The mixture was subjected to microwave irradiation (Initial Power=150W) and the reaction was conducted at 125°C for 5min. The mixture was then cooled to room temperature, filtered through a small plug of Celite and evaporated to dryness with the crude being analysed by GC-MS and/or GC-FID (methods described in Section 5.1). The residue obtained was dissolved in dichloromethane (20mL) and washed with water (3×20mL). The organic phase was dried over anhydrous Na2SO4, filtered and evaporated to a solid material or to a waxy residue. The reaction products were isolated and purified by column chromatography (Silicagel 60 (Merck), 0.063-0.200mm), using EtOAc/n-hexane or EtOAc/CH2Cl2 mixtures as the eluents (specified below for each compound).
References:
Carrilho, Rui M. B.;Damas, Liliana;Gonzalez, Andreia C. S.;Pereira, Mariette M.;Pineiro, Marta;Rodrigues, Fábio M. S. [Journal of Organometallic Chemistry,2020,vol. 923]
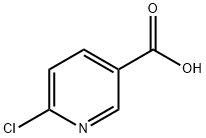
5326-23-8
546 suppliers
$5.00/1g

915921-01-6
10 suppliers
inquiry