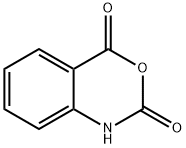
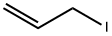N-ALLYLISATOIC ANHYDRIDE synthesis
- Product Name:N-ALLYLISATOIC ANHYDRIDE
- CAS Number:50784-07-1
- Molecular formula:C11H9NO3
- Molecular Weight:203.19
Yield:50784-07-1 84%
Reaction Conditions:
Stage #1: isatoic anhydridewith sodium hydride in N,N-dimethyl acetamide;mineral oil at 0; for 1 h;
Stage #2: allyl iodid in N,N-dimethyl acetamide;mineral oil at 20; for 1.5 h;
Steps:
2.1.a; 2.2.a a) 1-allyl-1 H-benzo[dl[1 ,3loxazine-2,4-dione:
Example 2.1 I Example 2.2: 1 O-allyl-3-cyclopentyl-2-isopropylpyrimidor45-b1guinoline- 45(3H1 OH)-dione I 3-cyclopentyl-2-isopropylpyrimidor45-b1guinoline-45(3H1 OH)dione : a) 1-allyl-1 H-benzo[dl[1 ,3loxazine-2,4-dione: 0.485 g of a 60% suspension of NaH in mineral oil (12.14 mmol) was suspended in 48.2 mLdry DMA, cooled to 0 °C and 2 g 1 H-benzo[d][1 ,3]oxazine-2,4-dione (90% technical grade,11.03 mmol) were added in portions and stirred for 1 hour. Then, 1.315 mL allyl iodide (2.41g, 14.34 mmol) were slowly added and stirred at room temperature for 1.5 hours. The reaction mixture was poured into 250 mL 0.1 M aqueous hydrochloric acid and stirred for 10 minutes. The resulting solid was filtered and washed with 60 mL water and 20 mL diethyl ether pentane (8:2) and dried under vacuum at 65 °C to yield 1.88 g 1-allyl-1 Hbenzo[d][1,3]oxazine-2,4-dione (9.25 mmol, 84%) as an off-white solid.
References:
WO2014/91446,2014,A1 Location in patent:Page/Page column 15; 16
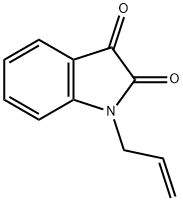
830-74-0
76 suppliers
$45.00/50mg

50784-07-1
26 suppliers
inquiry