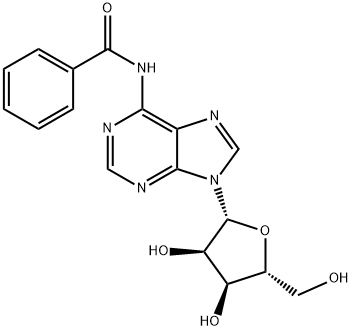
N-Benzoyladenosine synthesis
- Product Name:N-Benzoyladenosine
- CAS Number:4546-55-8
- Molecular formula:C17H17N5O5
- Molecular Weight:371.35
Yield:4546-55-8 90%
Reaction Conditions:
Stage #1:adenosine with pyridine;chloro-trimethyl-silane at 0; for 2 h;
Stage #2:benzoyl chloride at 25; for 14 h;
Steps:
1 benzoate 70
To a solution of33 (120g. 449 mmoi) in Py (1,0 L) is added TMSC1 (390g. 3.59 mol, 454 mL). After stirring at 0 °C for 2 hours, benzoyl chloride (316 g, 2.25 mol, 261 mL) is added dropwise and the mixture is stirred at 25 Q( for 14 hours before cooled to 0 °C. Water (240 mL) is then added and the mixture is stirred at 25 °C for 30 minutes before ammoniurn hydroxide (460 rnL) is added at 0 °C. After stirring for 2 hours, the mixture is concentrated to give 70 as a white solid (150 g. 90% yield)
References:
IMMUNE SENSOR, LLC;THE BOARD OF REGENTS OF THE UNIVERSITY OF TEXAS SYSTEM;ZHONG, Boyu;SUN, Lijun;WEI, Qi;DAI, Yuanwei;CHEN, Chuo;CHEN, Zhijian WO2017/161349, 2017, A1 Location in patent:Paragraph 0288

98-88-4
577 suppliers
$10.00/5g

4546-55-8
244 suppliers
$5.00/1g
14686-89-6
125 suppliers
$16.00/100mg

4546-55-8
244 suppliers
$5.00/1g
22331-21-1
76 suppliers
$70.00/10 g

4546-55-8
244 suppliers
$5.00/1g

