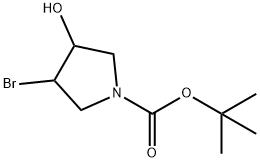
N-BOC-3-BROMO-4-HYDROXY-PYRROLIDINE synthesis
- Product Name:N-BOC-3-BROMO-4-HYDROXY-PYRROLIDINE
- CAS Number:1017782-17-0
- Molecular formula:C9H16BrNO3
- Molecular Weight:266.13
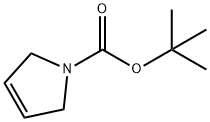
73286-70-1
290 suppliers
$6.00/5g

1017782-17-0
21 suppliers
inquiry
Yield:1017782-17-0 78%
Reaction Conditions:
with N-Bromosuccinimide in water;dimethyl sulfoxide at 0 - 25; for 4 h;Inert atmosphere;
Steps:
217.1 Step 1: tert-butyl 3-bromo-4-hydroxypyrrolidine-1-carboxylate
[00695] To a solution of 231-1 (2.0 g, 12 mmol, 1.0 eq) in DMSO (26 mL) and H20 (1.6 mL) at 0 C was added BS (2.7 g, 15 mmol, 1.3 eq). The reaction mixture was warmed to 25 C and stirred for 4 hr under N2. TLC (PE/EA = 3/1) showed a new spot was generated. H20 (10 mL) was added to quench the reaction, the aqueous phase was extracted with ethyl acetate (20 mL * 3). The combined organic phase was washed with brine (20 mL * 2), dried with anhydrous Na2S04, filtered and concentrated in vacuum. The residue was purified by silica gel chromatography to afford compound 231-2 (2.45 g, 78% yield). 1HNMR (400 MHz, CDC13) 4.34- 4.19 (m, 1H), 4.07 (d, J = 3.5 Hz, 1H), 3.89 (dd, J= 4.8, 13.1 Hz, 1H), 3.71- 3.54 (m, 2H), 3.25 (t, J= 12.3Hz, 1H), 1.33 (s, 9H).
References:
WO2018/204532,2018,A1 Location in patent:Paragraph 00695

24424-99-5
850 suppliers
$13.50/25G

1017782-17-0
21 suppliers
inquiry
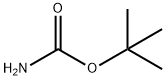
4248-19-5
380 suppliers
$5.00/5g

1017782-17-0
21 suppliers
inquiry