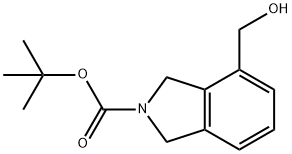
N-BOC-4-(HYDROXYMETHYL)ISOINDOLINE synthesis
- Product Name:N-BOC-4-(HYDROXYMETHYL)ISOINDOLINE
- CAS Number:1311254-56-4
- Molecular formula:C14H19NO3
- Molecular Weight:249.31
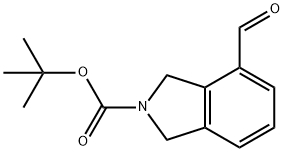
1049677-40-8
21 suppliers
$60.00/5mg

1311254-56-4
15 suppliers
$45.00/10mg
Yield: 213 mg
Reaction Conditions:
with methanol;sodium tetrahydroborate at 20;Cooling with ice;
Steps:
24 Preparation Example 24
Preparation Example 24 [0127] To a solution of 350 mg of 141 tert-butyl 4-formyl-1,3-dihydro-2H-isoindole-2-carboxylate in 11 mL of 43 methanol was added 134 mg of 148 sodium borohydride under ice-cooling, followed by stirring at room temperature overnight. To the reaction liquid were added 72 water and 83 ethyl acetate to carry out a liquid separation operation. The obtained organic layer was washed with saturated brine and then dried over anhydrous magnesium sulfate. The solvent was evaporated under reduced pressure to obtain 213 mg of 149 tert-butyl 4-(hydroxymethyl)-1,3-dihydro-2H-isoindole-2-carboxylate as a yellow oily substance
References:
Astellas Pharma Inc.;KAIZAWA, Hiroyuki;SUGITA, Mari;YAMAMOTO, Hirofumi;KAMIJO, Kazunori;TSUCHIYA, Kazuyuki;SEO, Ryushi;YAMAMOTO, Satoshi EP2615096, 2013, A1 Location in patent:Paragraph 0127
775545-06-7
10 suppliers
inquiry

1311254-56-4
15 suppliers
$45.00/10mg

24424-99-5
848 suppliers
$13.50/25G

1311254-56-4
15 suppliers
$45.00/10mg