
N-Boc-6-azaindole synthesis
- Product Name:N-Boc-6-azaindole
- CAS Number:370880-82-3
- Molecular formula:C12H14N2O2
- Molecular Weight:218.25
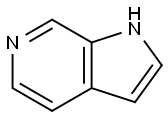
271-29-4
266 suppliers
$9.00/250mg

24424-99-5
820 suppliers
$13.50/25G

370880-82-3
27 suppliers
$62.00/100mg
Yield:370880-82-3 100%
Reaction Conditions:
with triethylamine at 0 - 20; for 3 h;
Steps:
17.1 D/ert-butyl lH-pyrrolo[2.3-c1pyridine-l-carboxylate
To a solution of 6-azaindoIe (6.02 g) and Et3N (14 mL) at 0°C was added (Boc)20 (16.50 mL) dropwise. The reaction mixture was stirred at room temperature for 3 h, washed with water and extracted with EtOAc. The organic layer was dried over anhydrous Na2S04 for 1 h and concentrated in vacuo. The residue was chromatographed with a silica gel column (eluting agent: 3:1 (v/v) PE/EA) to afford the title compound as transparent liquid (11.10 g, 100.00 %). The compound was characterized by the following spectroscopic data: 'HNMR(400 MHz, CDC13) ?: 1.70 (s, 9H), 6.59 (d, J =3.6 Hz, 1H), 7.49 (d, J = 5.3 Hz, 1H), 7.75 (d, .7=3.5 Hz, 1H), 8.40 (d, J= 5.3 Hz, 1H), 9.39 (s, 1H) ppm.
References:
WO2013/71697,2013,A1 Location in patent:Paragraph 00268

271-29-4
266 suppliers
$9.00/250mg
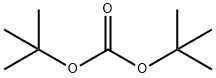
34619-03-9
26 suppliers
inquiry

370880-82-3
27 suppliers
$62.00/100mg
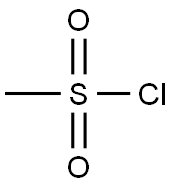
124-63-0
6 suppliers
$10.00/5g
![tert-butyl 2-hydroxy-2,3-dihydro-1H-pyrrolo[2,3-c]pyridine-1-carboxylate](/CAS/20180703/GIF/185139-01-9.gif)
185139-01-9
5 suppliers
inquiry

370880-82-3
27 suppliers
$62.00/100mg