
N-Carboxybenzyl GeMcitabine synthesis
- Product Name:N-Carboxybenzyl GeMcitabine
- CAS Number:138685-83-3
- Molecular formula:C17H17F2N3O6
- Molecular Weight:397.33

501-53-1
394 suppliers
$10.00/1g

95058-81-4
518 suppliers
$6.00/100mg

138685-83-3
19 suppliers
$174.90/5mg
Yield:138685-83-3 91%
Reaction Conditions:
Stage #1: gemcitabinewith pyridine;chloro-trimethyl-silane at 4 - 20; for 1.5 h;Inert atmosphere;
Stage #2: benzyl chloroformate in methanol at 20;Inert atmosphere;
Steps:
4-N-(Benzyloxycarbonyl)-2-deoxy-2,2-difluorocytidine (18)
A suspension of gemcitabine 1 (700 mg, 2.7 mmol) in dry pyridine (25 mL) was prepared and cooled to 4°C in an ice bath. Trimethylsilylchloride (3.5 mL, 27 mmol) was added dropwise under an argon atmosphere and the mixture was left to stir at room temperature. After 1.5 h,the solution was cooled to 4°C in an ice bath and benzyl chloroformate (2 mL, 14 mmol) was added dropwise. After 12 h the solution wascooled to 4°C in an ice bath, methanol (24 mL) was added slowly and the reaction mixture was stirred at room temperature overnight. Then themixture was evaporated to dryness. The residue was treated with saturated sodium bicarbonate (20 mL) and it was extracted with ethylacetate (3 x 40 mL). The combined organic extracts were dried over anhydrous magnesium sulfate, filtered and evaporated to dryness withrepeated coevaporation using toluene. The residue was dissolved in methanol and evaporated with silica gel (2 g). The crude product waspurified by silica gel column chromatography using dichloromethane-methanol (gradient from 100:1 to 10:1, v/v) as eluent to afford 18 (yield:961.6 mg, 91%).
References:
Trznadel, Roksana;Singh, Aleksandra;Kleczewska, Natalia;Liberska, Joanna;Ruszkowski, Piotr;Celewicz, Lech [Bioorganic and Medicinal Chemistry Letters,2019,vol. 29,# 18,p. 2587 - 2594] Location in patent:supporting information

501-53-1
394 suppliers
$10.00/1g

122111-03-9
627 suppliers
$8.00/25mg

138685-83-3
19 suppliers
$174.90/5mg
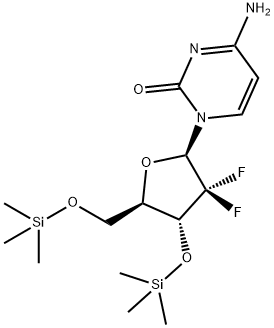
1155265-38-5
0 suppliers
inquiry

501-53-1
394 suppliers
$10.00/1g

138685-83-3
19 suppliers
$174.90/5mg

122111-03-9
627 suppliers
$8.00/25mg

138685-83-3
19 suppliers
$174.90/5mg

1155265-38-5
0 suppliers
inquiry

138685-83-3
19 suppliers
$174.90/5mg