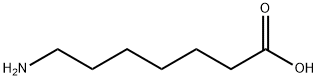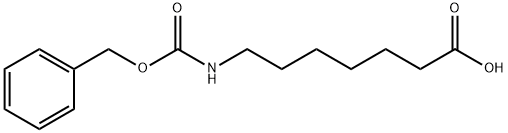
N-Cbz-7-aminoheptanoic acid synthesis
- Product Name:N-Cbz-7-aminoheptanoic acid
- CAS Number:23434-37-9
- Molecular formula:C15H21NO4
- Molecular Weight:279.33
Yield:23434-37-9 88%
Reaction Conditions:
with sodium hydroxide in lithium hydroxide monohydrate at 0 - 20; for 0.5 h;
Steps:
5 4.1.4. General procedure for the synthesis of 8-12
General procedure: To a cooled (0°C) solution of the appropriate amino acid (1 eq) in NaOH 2N, a solution of benzyl chloroformate (1.1 eq) was added dropwise. The mixture was kept under vigorous stirring for 30 min. The solution was then washed with Et2O, acidified to pH=2 with HCl 3 N and extracted with EtOAc. The organic phase was dried over Na2SO4 and evaporated under vacuo to give 8-12. 4.1.4.5
7-(((benzyloxy)carbonyl)amino)heptanoic acid (12)
White solid: 88% yield, 1H NMR (400 MHz, DMSO) δ 1.29-1.36 (m, 4H), 1.52-1.60 (m, 4H), 2.30-2.37 (m, 2H), 3.18 (t, 2H, J = 6 Hz), 5.09 (brs, 1H, exch D2O), 6.03 (s, 2H), 7.38-7.47 (m, 5H), 11.0 (brs, 1H, exch D2O); ESI-MS (m/z): 280 [M+H]+.
References:
Tarozzi, Andrea;Marchetti, Chiara;Nicolini, Benedetta;D'Amico, Massimo;Ticchi, Nicole;Pruccoli, Letizia;Tumiatti, Vincenzo;Simoni, Elena;Lodola, Alessio;Mor, Marco;Milelli, Andrea;Minarini, Anna [European Journal of Medicinal Chemistry,2016,vol. 117,p. 283 - 291]
673-66-5
141 suppliers
$6.00/250mg

23434-37-9
32 suppliers
inquiry

501-53-1
393 suppliers
$10.00/1g

23434-37-9
32 suppliers
inquiry