
N-Cyano-1-chloroforMaMidine synthesis
- Product Name:N-Cyano-1-chloroforMaMidine
- CAS Number:25816-30-2
- Molecular formula:C2H2ClN3
- Molecular Weight:103.51
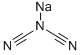
1934-75-4
270 suppliers
$6.00/25g

25816-30-2
9 suppliers
inquiry
Yield:-
Reaction Conditions:
with hydrogenchloride in water at -30 - 35; for 1.08333 h;
Steps:
9 Example 9 2, 4-DICHLORO-1, 3, 5-triazine
Example 9 2, 4-DICHLORO-1, 3, 5-triazine : Sodium DICYANAMIDE (LOG, 0.12 mol) is dissolved in water and added quickly to concentrated hydrochloric acid (60 mL) cooled to ABOUT-30 C. The slurry was stirred at that temperature for about 15 minutes and then warmed to 35 C for 5 minutes before being cooled to 4 C for 45 minutes. The white precipitate was then filtered, washed with small amounts of water, dried under vacuum for 24 hours. About 5G of N-cyanochloro- FORMAMIDINE was obtained : 1H NMR (DMSO-D6): 8 7.59 (s, 1H). To a solution of DMF (1.1 equivalent, 6.0 ML, 77 mmol) in dichloromethane, at room temperature, was added phosphorous oxychloride (1.0 equivalent, 6.5 ML, 70 mmol) and then, after about 10 minutes, 1.0 eq. OF N-CYANOCHLOROFORMAMIDINE (6.25g, 70 mmol) was added. The mixture was stirred overnight at room temperature and then washed 3 times with water and once with brine. The organic phase was dried over sodium sulfate, filtered, and evaporated under reduced pressure. The white solid (~4G) thus obtained wais identified as the 2, 4-DICHLORO-1, 3,5-triazine : LH NMR (CDC13) 6 8.88 (s, 1H).
References:
WO2004/72063,2004,A1 Location in patent:Page 33