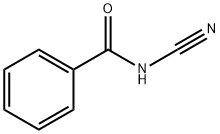
N-cyanobenzamide synthesis
- Product Name:N-cyanobenzamide
- CAS Number:15150-25-1
- Molecular formula:C8H6N2O
- Molecular Weight:146.15

108-86-1
506 suppliers
$10.00/5g
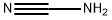
420-04-2
345 suppliers
$15.00/25g
201230-82-2
1 suppliers
inquiry

15150-25-1
25 suppliers
$57.50/250 mg
Yield: 85%
Reaction Conditions:
with tetrakis(triphenylphosphine) palladium(0);triethylamine in 1,4-dioxane at 65; under 1520.1 Torr; for 18 h;High pressure;
Steps:
General Procedure for the Aminocarbonylation Using CO gas
General procedure: Aryl iodide/bromide (0.5 mmol), cyanamide (3.0 equiv), Pd(PPh3)4 (5 mol%) and Et3N (1mmol) was taken up in 1,4-dioxane (10 mL) and the reaction mixture was placed inside a highpressure reactor. The reactor was pressurized with CO (2 atm) and heated for 18 h at 65 C for iodides and 85 C for bromides.
References:
Mane, Rajendra S.;Nordeman, Patrik;Odell, Luke R.;Larhed, Mats [Tetrahedron Letters,2013,vol. 54,# 50,p. 6912 - 6915] Location in patent:supporting information
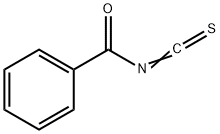
532-55-8
209 suppliers
$9.00/1g

15150-25-1
25 suppliers
$57.50/250 mg
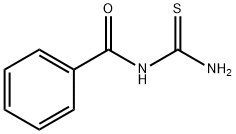
614-23-3
101 suppliers
$11.00/1g

15150-25-1
25 suppliers
$57.50/250 mg
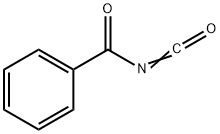
4461-33-0
97 suppliers
$13.00/1g

15150-25-1
25 suppliers
$57.50/250 mg
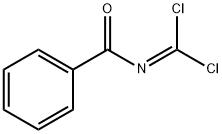
3911-55-5
0 suppliers
inquiry

15150-25-1
25 suppliers
$57.50/250 mg