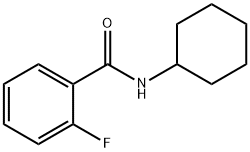
N-Cyclohexyl-2-fluorobenzaMide, 97% synthesis
- Product Name:N-Cyclohexyl-2-fluorobenzaMide, 97%
- CAS Number:2267-95-0
- Molecular formula:C13H16FNO
- Molecular Weight:221.27
Yield:2267-95-0 88%
Reaction Conditions:
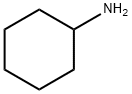
Stage #1: cyclohexylaminewith potassium carbonate in acetonitrile at 70;
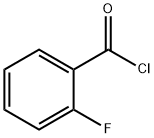
Stage #2: 2-Fluorobenzoyl chloride in acetonitrile at 70; for 4 h;
Steps:
A typical procedure for the synthesis of 1a:
General procedure: A mixture of propylamine (0.885 g, 15.0 mmol), K2CO3 (2.070 g, 15.0 mmol) andCH3CN (25 mL) in a 100-mL round-bottomed flask was stirred at 70 °C for a few minutes,and then 2-fluorobenzoyl chloride (1.585 g, 10.0 mmol) was added dropwise to the mixture(ca. 0.5 h). The obtained reaction mixture was heated for additional 4 h and then cooled toroom temperature, water (50 mL) was added and the mixture was extracted with ethyl acetate(3 × 50 mL). The combined organic phases were dried over MgSO4.The filtered solution wasconcentrated under reduced pressure, and the crude residue was purified by columnchromatography on silica gel with the use of petroleum ether/ethyl acetate (gradient mixtureratio from 20:1 to 4:1 in volume) to afford 1a as a pale yellow oil in 90% yield (1.635 g).1b ~ 1m were prepared in 2.0 mmol-scale of benzoyl chlorides.
References:
Chen, Qian;Wang, Yunpeng;Hua, Ruimao [Molecules,2019,vol. 24,# 20,art. no. 3773] Location in patent:supporting information

1759-68-8
22 suppliers
$30.00/20mg

2267-95-0
4 suppliers
inquiry
52833-63-3
27 suppliers
$81.06/250mg

2267-95-0
4 suppliers
inquiry
89-99-6
247 suppliers
$10.00/1g

2267-95-0
4 suppliers
inquiry