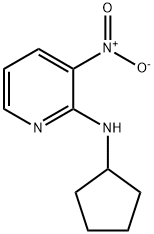
N-cyclopentyl-3-nitropyridin-2-amine synthesis
- Product Name:N-cyclopentyl-3-nitropyridin-2-amine
- CAS Number:952934-74-6
- Molecular formula:C10H13N3O2
- Molecular Weight:207.23
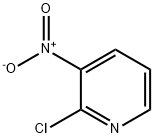
5470-18-8
439 suppliers
$6.00/5g
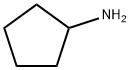
1003-03-8
212 suppliers
$10.00/1g

952934-74-6
14 suppliers
$137.84/1gm:
Yield:952934-74-6 81%
Reaction Conditions:
in tetrahydrofuran; for 18 h;Reflux;
Steps:
2.1 Step 1. Synthesis of N-cyclopentyl-3-nitropyridin-2-amine (C4)
Step 1.
Synthesis of N-cyclopentyl-3-nitropyridin-2-amine (C4)
Cyclopentanamine (2.7 g, 32 mmol) was added to a solution of 2-chloro-3-nitropyridine (5.0 g, 32 mmol) in tetrahydrofuran (200 mL), and the reaction mixture was stirred at reflux for 18 hours.
After removal of solvent under reduced pressure, the residue was purified via silica gel chromatography to give the product as a yellow solid. Yield: 5.5 g, 26 mmol, 81%. 1H NMR (400 MHz, CD3OD) δ 8.42 (dd, half of ABX pattern, J=8.3, 1.8 Hz, 1H), 8.40 (dd, half of ABX pattern, J=4.5, 1.8 Hz, 1H), 6.69 (dd, J=8.3, 4.5 Hz, 1H), 4.51-4.59 (m, 1H), 2.07-2.17 (m, 2H), 1.62-1.85 (m, 4H), 1.51-1.62 (m, 2H)
References:
US2014/235612,2014,A1 Location in patent:Paragraph 0200; 0201