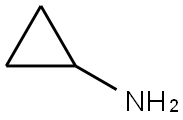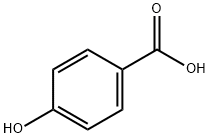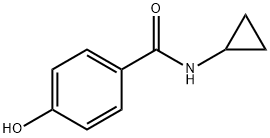
N-cyclopropyl-4-hydroxybenzamide synthesis
- Product Name:N-cyclopropyl-4-hydroxybenzamide
- CAS Number:860298-71-1
- Molecular formula:C10H11NO2
- Molecular Weight:177.2
Yield:860298-71-1 93%
Reaction Conditions:
with benzotriazol-1-ol;1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride;N-ethyl-N,N-diisopropylamine in N,N-dimethyl-formamide at 20; for 16 h;
Steps:
E
To a stirred solution of a primary or secondary amine (1 equivalent), a carboxylic acid (1.1-2.0 equivalents), 1-hydroxy-benzotriazole hydrate (HOBT) (1.1-2.0 equivalents) and diisopropylethylamine (DIPEA) or N-methylmorpholine (NMM) (1.5-3 equivalents) in CH2Cl2 or DMF (concentration -0.05-1.5M) was added l-[3-(dimethylamino)propyl]-3- ethylcarbodiimide hydrochloride (EDCI) (1.1-2.0 equivalents). The solution was stirred at room temperature for 1-3 days and concentrated in vacuo. In a standard work-up, the mixture was diluted with CH2Cl2 or EtOAc and washed consecutively with saturated aqueous NaHCO3 and brine. The organic layer was dried (Na2SO4 or MgSO4), filtered and concentrated under reduced pressure. The crude material was purified by flash column chromatography or by radial chromatography on silica gel.; Following general procedure E: a mixture of 4-hydroxybenzoic acid (2.76 g, 20.0 rnrnol), cyclopropyl amine (1.71 g, 30.0 mmol), EDCI (4.80 g, 25.0 mmol), HOBT (3.38 g, 25.0 mmol) and DPEA (3.87 g, 30.0 mmol) in DMF (20 mL) was stirred for 16 h affording N- cyclopropyl-4-hydroxy-benzarnide as a white solid (3.30 g, 93%).
References:
WO2007/22371,2007,A2 Location in patent:Page/Page column 25; 70