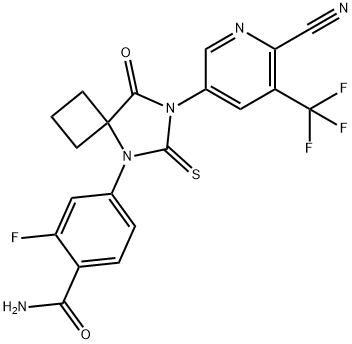
N-desmethyl?Apalutamide synthesis
- Product Name:N-desmethyl?Apalutamide
- CAS Number:1332391-11-3
- Molecular formula:C20H13F4N5O2S
- Molecular Weight:463.41

1332391-04-4
22 suppliers
inquiry

1332391-11-3
32 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: 4-(7-(6-cyano-5-(trifluoromethyl)pyridin-3-yl)-8-oxo-6-thioxo-5,7-diazaspiro[3.4]octan-5-yl)-2-fluorobenzoic acidwith oxalyl dichloride;N,N-dimethyl-formamide in dichloromethane at 20; for 4 h;
Stage #2: with ammonia in 1,4-dioxane at 20;
Steps:
34
To a suspension of 4-(7-(6-cyano-5-(trifluoromethyl)pyridin-3-yl)-8-oxo-6-thioxo-5,7- diazaspiro[3.4]octan-5-yl)-2-fluorobenzoic acid (Compound 224, 500 mg, 1.08 mmol) in DCM was added DMF (cat., 0.1 mL), followed by oxalyl chloride (0.14 mL, 1.61 mmol). The mixture was stirred at room temperature for 4h then concentrated in vacuo to produce a yellow residue that was further dried on a high vacuum pump. Ammonia (0.5M in dioxane, 40 mL, 20 mmol) was directly added to the residue and the mixture was stirred at room temperature overnight. MeOH was added and the mixture was absorbed onto silica gel and purified by flash chromatography (50 to 100% EtOAc/Hexanes) to afford impure desired product that was repurified by reverse phase HPLC (acetonitrile/water:TFA). The fractions containing the desired compound were combined, acetonitrile was removed in vacuo, and the remaining aqueous layer was treated with a saturated solution of sodium bicarbonate. The aqueous layer was extracted with DCM (3x), the organics were combined, dried over sodium sulfate, and evaporated to dryness to afford 100 mg of 4-(7-(6-cyano-5-(trifluoromethyl)pyridin-3-yl)-8- oxo-6-thioxo-5,7-diazaspiro[3.4]octan-5-yl)-2-fluorobenzamide as a white solid. lK NMR (300 MHz, DMSO-de) δ 9.21 (s, 1H), 8.75 (s, 1H), 7.95 (s, 1H), 7.87 (t, 1H), 7.80 (s, 1H), 7.46 (dd, 1H), 7.37 (dd, 1H), 2.69-2.62 (m, 2H), 2.55-2.47 (m, 2H), 2.00 (m, 1H), 1.58 (m, 1H).
References:
WO2011/103202,2011,A2 Location in patent:Page/Page column 200; 201
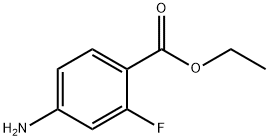
73792-06-0
68 suppliers
$27.00/250mg

1332391-11-3
32 suppliers
inquiry

573762-62-6
222 suppliers
inquiry

1332391-11-3
32 suppliers
inquiry
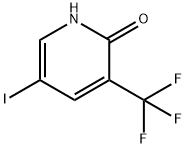
887707-23-5
108 suppliers
$28.00/1g

1332391-11-3
32 suppliers
inquiry
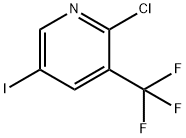
887707-25-7
118 suppliers
$20.00/1g

1332391-11-3
32 suppliers
inquiry