
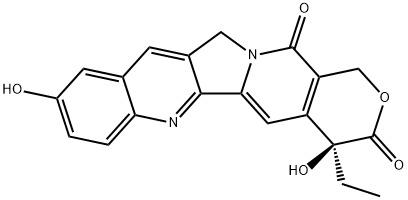
N-DESMETHYL TOPOTECAN synthesis
- Product Name:N-DESMETHYL TOPOTECAN
- CAS Number:190710-79-3
- Molecular formula:C22H21N3O5
- Molecular Weight:407.42
19685-09-7
436 suppliers
$16.00/100mg

593-51-1
546 suppliers
$21.00/100g

190710-79-3
24 suppliers
$100.00/100ug
Yield:190710-79-3 50 %
Reaction Conditions:
with formaldehyd;acetic acid in water at 80;Sealed tube;
Steps:
21.1 Step-1: Synthesis of (S)-4-Ethyl-4,9-dihydroxy-10-((methylamino)methyl)-l,12-dihydro-14H- pyrano[3',4':6,7]indolizino[l,2-b]quinoline-3,14(4H)-dione (Int-2)
To a stirred solution of (.S>4-cthyl-4,9-dihydroxy- 1 , 12-di hydro- 14//-pyrano[3',4':6,7|indoli/.ino[ 1 ,2- /?|qui no line-3, 14(4//)-dionc (Int-1, 2.0 g, 5.4 mmol, 1.0 eq.) and methyl amine hydrochloride (255 mg, 8.2 mmol, 1.5 eq.) in acetic acid (20 mL) was added 37% solution of formaldehyde (197 mg, 6.59 mmol, 1.2 eq.) at room temperature. The reaction mixture was heated to 80°C and stirred for 2 h in a sealed tube. Progress of the reaction was monitored by TLC. After completion of the reaction, solvents were evaporated under reduced pressure to get crude compound. The crude compound was basified with aq. ammonia until the pH reached to 9, filtered the solid and washed with water, dried under vacuum to afford Int-2 (1.7 g, 50%). 1H NMR (400 MHz, DMSO-ife) d 8.70 (s, 1H) 7.97 (d, J 9o.l2i9n Hz, 1H) 7.40 (d, J 9o.l2i9n Hz, 1H) 7.24 (s, 1H) 5.41 (s, 2H) 5.22 (s, 2H) 5.20 (br s, 1H) 4.37 (s, 2H) 2.46 (s, 3H) 1.82 - 1.93 (m, 3H) 0.88 (br t, / = 6.85 Hz, 3H). LCMS: 408.4 [M+H]+.
References:
WO2022/204184,2022,A1 Location in patent:Page/Page column 152-153

50-00-0
872 suppliers
$10.00/25g

19685-09-7
436 suppliers
$16.00/100mg

74-89-5
7 suppliers
$13.44/25ML

190710-79-3
24 suppliers
$100.00/100ug