
n-Docosanol synthesis
- Product Name:n-Docosanol
- CAS Number:661-19-8
- Molecular formula:C22H46O
- Molecular Weight:326.6

112-85-6
344 suppliers
$5.00/10g

661-19-8
376 suppliers
$5.00/250mg
Yield:661-19-8 87.6%
Reaction Conditions:
with sodium tetrahydroborate;iodine in tetrahydrofuran at 0 - 30;Reagent/catalyst;
Steps:
2; 3 PREPARATION OF DOCOSANOL:
Sodium borohydride (2.9 g) was suspended in THF (100 ml) at 20-30 °C and cooled to 0-5 °C. Thereafter, added a solution of iodine (7.5 g) dissolved in THF (30 ml) over a period of 1-2 h at 0-5 °C followed by a solution of docosanoic acid (20 g) dissolved in THF (140 ml) in 30-40 min. The reaction mixture was stirred for 2-4 h at 20-30 °C. After completion of reaction, the reaction mixture was carefully quenched with dil. HC1 (30 ml, 3N) at 20-30 °C and evaporated the solvent under reduced pressure (40-50 mm Hg) at 40-45 °C to get a turbid oily mass. To the above residue DM water (200 ml) was added and extracted the product in methyl-tert-butyl ether (300 ml). The organic extract was washed with an aqueous solution of 10% w/w sodium hydroxide (100 ml), an aqueous solution of 5% w/w sodium metabisulfite and finally with DM water (100 ml). Thereafter, the organic extract was concentrated under reduced pressure (20-40 mm Hg) at 40-50 °C to get a white colour residue. The above residue was dissolved in n- heptane (100 ml) at 40-45 °C and stirred for an hour at 20-25 °C. The resulting solid was filtered and dried under reduced pressure (10-20 mm Hg) to afford 16.8 g of the title compound. Yield: 87.6 % of theory Chromatographic purity (by GC): >98 %
References:
WO2018/220504,2018,A1 Location in patent:Page/Page column 8; 9

2433-97-8
109 suppliers
$43.00/250mg

661-19-8
376 suppliers
$5.00/250mg
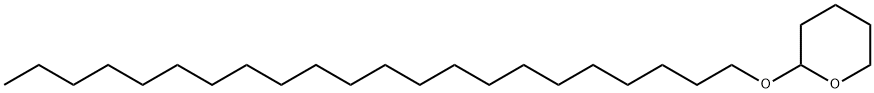
184678-19-1
1 suppliers
inquiry

661-19-8
376 suppliers
$5.00/250mg

57402-36-5
19 suppliers
$60.00/500mg

661-19-8
376 suppliers
$5.00/250mg

42449-18-3
0 suppliers
inquiry

661-19-8
376 suppliers
$5.00/250mg