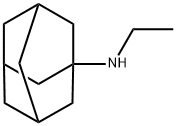
n-ethyl-1-adamantanamin synthesis
- Product Name:n-ethyl-1-adamantanamin
- CAS Number:3717-44-0
- Molecular formula:C12H21N
- Molecular Weight:179.3
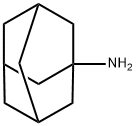
768-94-5
398 suppliers
$41.00/25g
56553-60-7
622 suppliers
$10.00/5g

3717-44-0
14 suppliers
$285.00/500mg
![Tricyclo[3.3.1.13,7]decan-1-amine, N,N-diethyl-](/CAS/20210305/GIF/3788-37-2.gif)
3788-37-2
0 suppliers
inquiry
Yield:3788-37-2 73.4% ,3717-44-0 20.5%
Reaction Conditions:
in acetonitrile at 60; for 15 h;
Steps:
4. General Procedure of Experiment in Table 1.
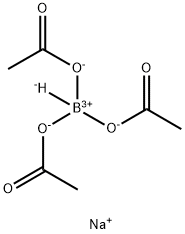
General procedure: All reactions were carried out according to the procedure written below unless otherwise noted. The starting materials, aliphatic amines, (0.08 mmol) in dry acetonitrile (1.6 mL) was treated with NaBH(OAc)3 (250 mg, 1.2 mmol) at 60 °C for 15 h. The reaction were worked up in the same manner as described in above section. Compound 7a: 1H-NMR (500 MHz, CDCl3) d 2.52 (4H, q, J = 7.2 Hz), 2.40 (2H, d, J = 7.8 Hz), 1.25 (32H, m), 1.02 (6H, t, J = 7.2 Hz), 0.88 (3H, t, J = 7.0 Hz); HRMS (ESI-TOF) m/z 326.3774 (Calcd for C22H48N 326.3781 [M+H]+).
References:
Tamura, Satoru;Sugawara, Aoi;Sato, Erika;Sato, Fuka;Sato, Keigo;Kawano, Tomikazu [Tetrahedron Letters,2020,vol. 61,# 22,art. no. 151919] Location in patent:supporting information
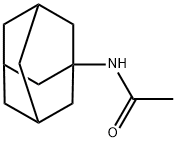
880-52-4
187 suppliers
$13.00/5g

3717-44-0
14 suppliers
$285.00/500mg

768-94-5
398 suppliers
$41.00/25g

64-19-7
1537 suppliers
$10.00/25ML

880-52-4
187 suppliers
$13.00/5g

3717-44-0
14 suppliers
$285.00/500mg
59987-81-4
1 suppliers
inquiry

3717-44-0
14 suppliers
$285.00/500mg
![Tricyclo[3.3.1.13,7]decan-1-amine, N-chloro-N-ethyl-](/CAS/20210305/GIF/34913-37-6.gif)
34913-37-6
0 suppliers
inquiry
935-56-8
186 suppliers
$34.85/5g

768-94-5
398 suppliers
$41.00/25g

3717-44-0
14 suppliers
$285.00/500mg