
N-Ethyl-3-Nitro-2-Pyridinamine synthesis
- Product Name:N-Ethyl-3-Nitro-2-Pyridinamine
- CAS Number:26820-65-5
- Molecular formula:C7H9N3O2
- Molecular Weight:167.17
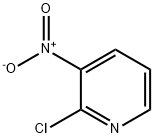
5470-18-8
434 suppliers
$6.00/5g
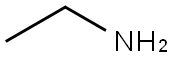
75-04-7
385 suppliers
$10.00/20mg

26820-65-5
24 suppliers
$55.00/250mg
Yield: 96%
Reaction Conditions:
in methanol at 90; for 0.25 h;Microwave irradiation;
Steps:
1 6.1.1. Ethyl-(3-nitropyridin-2-yl)amine (4)
A suspension of 2-chloro-3-nitropyridine (0.5 g, 3.15 mmol) in a2 M solution of ethylamine in MeOH (4.0 ml, 7.9 mmol) was irradiatedin a microwave oven at 90 C for 15 min. The reaction mixturewas then evaporated in vacuo, suspended in water, and extractedwith CH2Cl2 (3 20 ml). The organic layers were evaporated invacuo to give 4 (0.5 g, 96%) as a yellow solid: mp 83 C; 1H NMR(DMSOd6) d 1.17 (t, J = 7.00 Hz, 3H, CH2CH3), 3.50-3.65 (m, 4H,CH2CH3), 6.70 (dd, J = 4.0 and 8 Hz, 1H, pyridine CH), 8.35-8.45(m, 2H, pyridine CH).
References:
Sancineto, Luca;Iraci, Nunzio;Barreca, Maria Letizia;Massari, Serena;Manfroni, Giuseppe;Corazza, Gianmarco;Cecchetti, Violetta;Marcello, Alessandro;Daelemans, Dirk;Pannecouque, Christophe;Tabarrini, Oriana [Bioorganic and Medicinal Chemistry,2014,vol. 22,# 17,p. 4658 - 4666]

5470-18-8
434 suppliers
$6.00/5g

26820-65-5
24 suppliers
$55.00/250mg