N-ETHYL-4'-NITROACETANILIDE synthesis
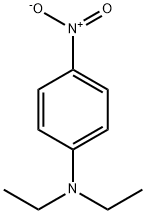
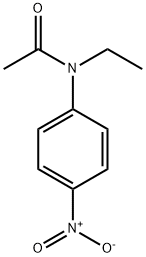
- Product Name:N-ETHYL-4'-NITROACETANILIDE
- CAS Number:1826-56-8
- Molecular formula:C10H12N2O3
- Molecular Weight:208.21
Yield:1826-56-8 85%
Reaction Conditions:
with sodium chlorate in lithium hydroxide monohydrate;acetonitrile at 60; under 760.051 Torr; for 1.5 h;Green chemistry;
Steps:
General procedure for the oxidation of tertiary amines to corresponding amides:
General procedure: A solution of (4-nitrophenyl) morpholine (1.04 g, 5 mmol) in CH3CN (10mL) was added into a 100mL three-port roundbottomflask, and it was stirred under CO2 atmosphere at 50°C. Then a solution of sodium chlorite (morethan 80%, 1.69 g, 15mmol) in 5 mL water was added in 20 min. TLC and HPLC showed that the reactionwas completed in 2.5 h. The reaction was quenched with aqueous saturated sodium sulfite. The mixture wasextracted by DCM (3×30 mL). The combined organic solution was dried over anhydrous sodium sulphate.Removal of all volatiles heft a residue, which was purified by flash chromatography on silica gel(hexane/EtOAc, from 5:1 to 3:1) to give 1.05g (95% yield) of 2a as a light-yellow solid.
References:
Liu, Chaoyang;Sun, Haozhou;Qin, Cheng;Yang, Tiannuo;Zhang, Wenxian;Zhou, Yuan;Li, Yani;Jia, Zheng Robert;Chu, Changhu [Synlett,2022,vol. 33,# 10,p. 993 - 997] Location in patent:supporting information
350-46-9
518 suppliers
$10.60/1gm:

1826-56-8
4 suppliers
inquiry

529-65-7
14 suppliers
$435.00/1000g

1826-56-8
4 suppliers
inquiry