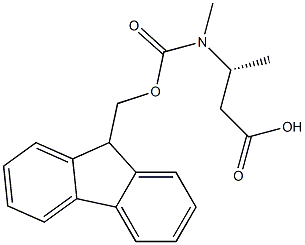
N-Fmoc-(R)-3-(methylamino)butanoic acid synthesis
- Product Name:N-Fmoc-(R)-3-(methylamino)butanoic acid
- CAS Number:1460306-60-8
- Molecular formula:C20H21NO4
- Molecular Weight:339.39
![Butanoic acid, 3-[[(9H-fluoren-9-ylmethoxy)carbonyl]methylamino]-, 2-propen-1-yl ester, (3R)-](/CAS/20210305/GIF/1460306-64-2.gif)
1460306-64-2
0 suppliers
inquiry

1460306-60-8
18 suppliers
inquiry
Yield:1460306-60-8 34%
Reaction Conditions:
with tetrakis(triphenylphosphine) palladium(0);1,3-dimethylbarbituric acid in dichloromethane;ethyl acetate at 20; for 3 h;
Steps:
Synthesis of (R)-3-((((9H-Fluoren-9-yl)methoxy)carbonyl)(methyl)amino)butanoic acid (40; Fmoc-NMe-33-homoDAIa-OH)
A degassed soln of 39 (18.3 g, 48.2 mmol) in CH2CI2 (175 mL) / EtOAc (210 mL) was treated with Pd(PPh3)4 (0.9 g, 0.77 mmol) and 1 ,3-dimetylbarbituric acid (9.04 g, 57.9 mmol) for 3 h at rt. The volatiles were evaporated. FC (CH2CI2/MeOH 100:0 to 80:20) afforded 40 (7.55 g, 46%) and impure material which was further purified by prep. HPLC (method 1 d) to give more 40 (5.61 g, 34%).Data of 40: C20H2iNO4 (339.4). LC-MS (method 1 a): Rt = 2.03 (96), 340.1 ([M+H]+). H-NMR (DMSO-de): 12.2 (br. s, 1 H); 7.89 (d, J = 7.4, 2 H); 7.65 (br. s, 2 H); 7.41 (t, J = 7.4, 2 H); 7.33 (t, J = 7.3, 2 H); 4.40 - 4.24 (m, 4 H), 2.67 (s, 3 H); 2.45- 2.30 (br. m, 2 H); 1.37 (br. d, 3 H).
References:
WO2013/139697,2013,A1 Location in patent:Page/Page column 129