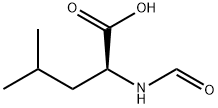
N-FORMYL-DL-LEUCINE synthesis
- Product Name:N-FORMYL-DL-LEUCINE
- CAS Number:5338-45-4
- Molecular formula:C7H13NO3
- Molecular Weight:159.18
Yield:5338-45-4 54%
Reaction Conditions:
with acetic anhydride at 20; for 91 h;
Steps:
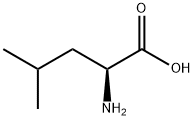
Leucine derivative
Leucine (2.18 g, 16.63 mmol) was dissolved in formic acid (10 mL), was added acetic anhydride (3.0 eq, 5.11 mL) and allowed to stirred 91 hours at room temperature the reaction. After completion of the reaction, the reaction mixture was concentrated under reduced pressure, recrystallized by adding methanol and ethyl acetate, the corresponding formamide (4a) was obtained (1.43 g, 8.96 mmol, 54%). Compound 4a (1.43 g, 8.99 mmol), and by DMF (20 mL) in benzyl bromide (1.5 eq, 1.70 mL) and potassium carbonate (3.2 eq, 3.96 g) and, then stirred at room temperature for 22 hours the reaction It was. After completion of the reaction, water is added to inactivate the reaction mixture, ethyl acetateIn was extracted. The organic layer was washed with saturated brine, dried over magnesium sulfate, filtered, and subjected to concentration under reduced pressure. The residue was purified by silica gel chromatography (hexane: ethyl acetate = 3: 2) to give the corresponding benzyl ester (4b) was obtained (1.40 g, 5.62 mmol, 63%). Compound 4b (825.3 mg, 3.31 mmol) was added in DCM (4 mL), the mixture obtained PhOPOCl2 (1.8eq, 890 μL) and pyridine (5.0 eq, 1.34 mL) was added, stirred for 2 hours the reaction went. OppositeAfter the response was finished, water was added to the reaction mixture, and the mixture was extracted with ethyl acetate. The organic layer, hydrochloric acid, successively washed sodium bicarbonate, water, and saturated brine, and dried over magnesium sulfate, filteredAfter, it was concentrated under reduced pressure. The residue was purified by silica gel chromatography (hexane: ethyl acetateLe = 3: 1) to give the isonitrile (4) (426.0 mg, 1.84 mmol, 56%). yellowColor oil.
References:
JP2015/42622,2015,A Location in patent:Paragraph 0127