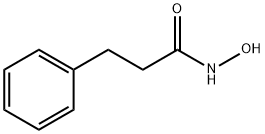
N-HYDROXY-3-PHENYL-PROPIONAMIDE synthesis
- Product Name:N-HYDROXY-3-PHENYL-PROPIONAMIDE
- CAS Number:17698-11-2
- Molecular formula:C9H11NO2
- Molecular Weight:165.19
104-53-0
287 suppliers
$6.00/5g

17698-11-2
6 suppliers
inquiry
Yield: 86%
Reaction Conditions:
with menadione;hydroxylamine hydrochloride;water;silver nitrate;triethylamine in tetrahydrofuran;ethanol at 20; for 0.5 h;Irradiation;Green chemistry;Angeli-Rimini Hydroxamic Synthesis;
Steps:
General procedure for the photosynthesis of hydroxamic acids 2a-j
General procedure: To an aqueous solution of AgNO3 (1.7 mg, 0.01 mmol, 1 mol%) in water (6 mL),vitamin K3 (2.6 mg, 0.015 mmol, 1.5 mol%) dissolved in ethanol (2 mL) wasadded, and then an appropriate aldehyde (1 mmol) dissolved in THF (2 mL) wasadded to the reaction mixture followed by the addition of NH2OHHCl (69 mg,1 mmol) and Et3N (0.14 mL, 1 mmol). The final solvent ratio (H2O:EtOH:THF)was (3:1:1). The mixture was stirred at room temperature for 30 min under visiblelight irradiation using a halogen lamp (HALOPAR 20 75 W 230 V 30-GU10;Osram, Italy). The reaction was monitored by TLC. After completion of thereaction, EtOAc (30 mL) was added. The aqueous layer was collected andconcentrated under reduced pressure at 25 C to obtain pure sample containing Agnanoparticles that was characterized by UV-Vis and TEM analyses. The organicphase was collected, dried over anhydrous MgSO4 and filtered. The solvent wasevaporated under vacuum using a rotatory evaporator and then the residue was purified by a short plug of silica gel using (Hexane:EtOAc, 1:1) to obtainhydroxamic acids 2a-j in 81-92% yield.
References:
Mohamed, Yasser M. A.;Attia, Yasser A.;Solum, Eirik Johansson [Research on Chemical Intermediates,2018,vol. 44,# 12,p. 7173 - 7186]
501-52-0
505 suppliers
$14.14/1gm:

17698-11-2
6 suppliers
inquiry
645-45-4
346 suppliers
$15.00/5g

17698-11-2
6 suppliers
inquiry

103-25-3
267 suppliers
$9.00/1g

17698-11-2
6 suppliers
inquiry
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

17698-11-2
6 suppliers
inquiry