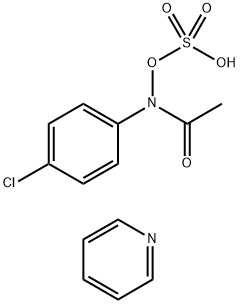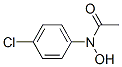
N-hydroxy-4-chloroacetanilide synthesis
- Product Name:N-hydroxy-4-chloroacetanilide
- CAS Number:1503-91-9
- Molecular formula:C8H8ClNO2
- Molecular Weight:0
Yield:1503-91-9 89%
Reaction Conditions:
with Sodium hydrogenocarbonate in diethyl ether at 0;Inert atmosphere;
Steps:
N-(4-chlorophenyl)-N-hydroxyacetamide (1f)
To a stirred suspension of N- ( 4-chlorophenyl ) hydroxylamine (Sit) (l.OOg, 6.97 mmol, l.OOequiv) and NaHCO3(0.700 g, 8.36 mmol, 1.20 equiv) in Et20 (20.0mL, 0.349 M ) at 0 °C under 2 was slowly added a solution of acetyl chloride (0.660 g, 8.36 mmol, 1.20 equiv) in Et2<3 (20.0 niL, 0.418 M) via a syringe pump(at a rate of lO.OmL/h). After the addition was complete, the reaction mixture was filtered through a short pad of celite and the celite was washed with EtOAc. The organic layers were combined and concentratedin vacuo. Recrystallization from Et20/hexanes afforded the title compound as a yellow solid (1.15 g, 6.20 mmol, 89% yield). Rf= 0.13 (hexanes/EtOAc 4:l(v/v)). NMR Spectroscopy: XH NMR (400MHz, (CD3)2SO, δ) : 10.72 (s, 1H), 7.67 (d, J=8.9 Hz, 2H) , 7.44-7.38 (m, 2H) , 2.21 (s, 3H) . 13C NMR (100 MHz, (CD3)2SO, δ) : 170.1, 140.5, 128.3, 128.1, 121.4, 22.5. Mass Spectrometry: HRMS (ESI-TOF) (m/z) : calcd for C8H9NO2CI ( [M + H]+)/ 186.0322, found, 186.0321.
References:
WO2016/57931,2016,A1 Location in patent:Page/Page column 70-71

100-00-5
12 suppliers
$14.00/25g

1503-91-9
2 suppliers
inquiry

1795-83-1
2 suppliers
inquiry

1503-91-9
2 suppliers
inquiry