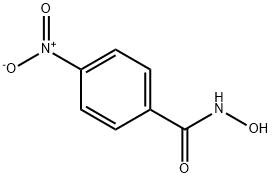
N-hydroxy-4-nitrobenzamide synthesis
- Product Name:N-hydroxy-4-nitrobenzamide
- CAS Number:1613-76-9
- Molecular formula:C7H6N2O4
- Molecular Weight:182.13
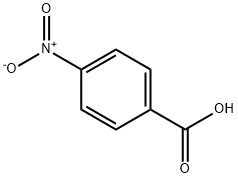
62-23-7
712 suppliers
$14.00/25g

1613-76-9
3 suppliers
inquiry
Yield:1613-76-9 70%
Reaction Conditions:
with hydroxylamine hydrochloride;1,1'-carbonyldiimidazole in N,N-dimethyl-formamide; for 20 h;
Steps:
Method a
General procedure: Acid (4 mmol) was dissolved in DMF (2 mL), and CDI (0.65 g, 4 mmol) in DMF (2 mL) was slowly added. After 2 h hydroxylamine hydrochloride (0.56 g, 8 mmol; as well as 0.67 g, 8 mmol of N- or O-methylated hydroxylamine hydrochloride) was added in one portion and mixture was shortly stirred until salt disappearance and incubated for additional 18 hours. The solution was poured into water, extracted with ethyl acetate and dried over Na2SO4. The solvent was removed at reduced pressure, and the oil was chromatographed on SiO2, eluting with CHCl3/EtOH, to give the raw product that was finally purified by washing with appropriate organic solvents. Yields 30-70 %.
References:
Kozlov, Maxim V.;Kleymenova, Alla A.;Romanova, Lyudmila I.;Konduktorov, Konstantin A.;Smirnova, Olga A.;Prasolov, Vladimir S.;Kochetkov, Sergey N. [Bioorganic and Medicinal Chemistry Letters,2013,vol. 23,# 21,p. 5936 - 5940] Location in patent:supporting information
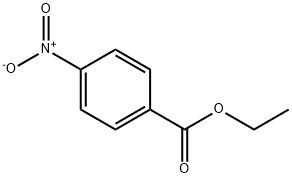
99-77-4
214 suppliers
$12.00/1mg

1613-76-9
3 suppliers
inquiry
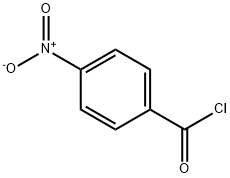
122-04-3
11 suppliers
$15.00/1g

1613-76-9
3 suppliers
inquiry

619-50-1
182 suppliers
$17.00/25g

1613-76-9
3 suppliers
inquiry

619-73-8
568 suppliers
$5.00/10g

1613-76-9
3 suppliers
inquiry