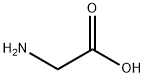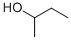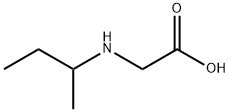
N-isobutyrylglycine synthesis
- Product Name:N-isobutyrylglycine
- CAS Number:58695-42-4
- Molecular formula:C6H13NO2
- Molecular Weight:131.17
Yield:-
Reaction Conditions:
with 1-hydroxytetraphenylcyclopentadienyl(tetraphenyl-2,4-cyclopentadien-1-one)-μ-hydrotetracarbonyldiruthenium(II);2,2,2-trifluoroethanol at 90; for 24 h;Schlenk technique;Inert atmosphere;
Steps:
3 General reaction conditions
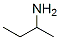
General procedure: General procedure, 0.2 or 0.5 mmol 1, 1 - 4 ml or 0.6 - 2 mmol 2, neat or 2d/tol as solvent, 1 mol % Cat 2, 18 - 24 h, 90 °C, isolated yields are shown, unless otherwise specified; "yields are base crude H MNR and mass balance; 6100 °C. For details see Table 4 and 5. After exploring the reactivity of various cc-amino acids with ethanol (2a) and isopropanol (2b), diverse alcohols were tried (Scheme 2, Table 4). As previously shown, MeOH (2c) or CF3CH2OH (2d) did not react with la, probably because they were not prone to be dehydrogenated under the present reaction conditions, which allowed for the possibility of using 2c or 2d as solvent. For other alcohol substrates such as 1-butanol (2e), cyclopropylmethanol (2f) and 2-chloroethanol (2g), employed in the reaction with la, resulted in quantitative yield of 3ea, 55 % of 3af, and 71 % of 3ag, respectively (Table 4, entry 3 - 5). Also, benzyl alcohol (3h) and 4- chlorobenzyl alcohol (3i) were successfully applied to benzylate la with good yields of 68 % and 82 % (Table 4, entry 6 and 7). Here it needs to be pointed out that, the chloro- group on 3ag and 3ai would allow the further functionalization of the obtained amino acid derivatives. Subsequently, the use of other amino acids such as phenylalanine (Id) and glycine (lg) were examined. Id reacted with 1-butanol (2e) to give 84 % yield of 3de (Table 4, entry 8). Surprisingly, instead of forming a 6-membered heterocycle, the reaction of Id with 1,5-pentane-diol (2k) gave a 35% yield of the di-alkylated 3dk as the major product (Table 4, entry 9). The introduced 2-hydroxyl groups on 3dk can not only be exploited for further functionalization, but also dramatically increase the hydrophilicity of the product. Next, the functionalization of lg with various alcohols was further explored. Upon reaction with 3h the di-benzylation product 3gh was obtained in 52 % yield while when secondary alcohol 2-butanol (21) was employed to alkylate lg, selective formation of mono-alkylated product 3gl was obtained in quantitative yield (Table 4, entry 10 and 12). Table 4: Direct N-alkylation of free amino acid (1) with various alcohols (2). General reaction conditions: General procedure, 0.2 or 0.5 mmol 1, 0.6 - 2 mmol or 2 - 5 ml 2, neat or with solvent (amount shown in the table), 1 mol % Cat 2, 16 - 24 h, 90 or 100°C, isolated yields in parenthesis; "Conversion and selectivities are based on crude H NMR. After this, the possibility of mono-alkylation with primary alcohols was also investigated. Using 4 eq. of 1-pentanol (lm), lg was successfully di- alkylated giving 90 % of 3gm. When the amount of lm was decreased to 1.2 eq., 46 % mono-alkylated product 3gm' was obtained, only giving 29 % of the di-alkylated product 3gm (Table 4, entry 15 and 16). As 3gm' is more basic than 3g, without enough steric hindrance on the nitrogen atom, the second alkylation step occurred immediately once 3gm' formed. Thus, mono-N- alkylated amino acids can be obtained from primary amino acids and primary alcohols, but with low selectivity. Long-chain N-alkylated amino acids are widely used in surfactants, among which N-alkylated amino acids are not well studied because they are relatively difficult to be synthetized9. Envisioning the possibility of easily synthetizing long-chain N-alkylated amino acids with our methodology, 1- nonanol (2j) was selected to react with Id and lg. Di-alkylated compounds 3dj and 3gj were selectively obtained with good to excellent yields of 75 % and 91 %, respectively (Table 4, entry 9 and 13). The reaction also readily proceeded with 1-dodecanol (In) and lg as substrate, obtaining the corresponding product 3gn in an excellent yield of 92 % (Table 4, entry 17).
References:
WO2018/178397,2018,A1 Location in patent:Page/Page column 17-19; 26; 28-31; 45; 46