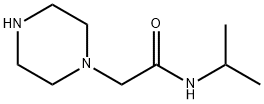
N-ISOPROPYL-1-PIPERAZINEACETAMIDE synthesis
- Product Name:N-ISOPROPYL-1-PIPERAZINEACETAMIDE
- CAS Number:39890-42-1
- Molecular formula:C9H19N3O
- Molecular Weight:185.27
![1-Piperazinecarboxylic acid, 4-[2-[(1-methylethyl)amino]-2-oxoethyl]-, 1,1-dimethylethyl ester](/CAS/20210305/GIF/1051655-63-0.gif)
1051655-63-0
0 suppliers
inquiry

39890-42-1
44 suppliers
$45.00/50mg
Yield:39890-42-1 4.41 g
Reaction Conditions:
with hydrogenchloride in ethyl acetate; for 3 h;
Steps:
47.2 Step 2:
N-isopropyl-2-(piperazin-1-yl)acetamide
The crude step 1 product was dissolved in ethyl acetate (300 mL).
Into this stirred solution was bubbled hydrogen chloride for approximately five minutes.
After another two hours the reaction was analyzed by TLC and found to still contain a minor portion of N-tert-butoxycarbonyl protected starting material.
The reaction mixture was saturated with hydrogen chloride gas as before and stirred for another one hour.
The mixture was then concentrated and the residue was partitioned between chloroform (?100 mL) and aqueous sodium carbonate solution (?200 mL).
The organic layer was combined with additional extracts (chloroform, 5*?100 mL), dried (Na2SO4) and concentrated to afford crude product as an off-white solid.
This material was subjected by automated flash chromatography (Combiflash system; 10% methanol in chloroform until the impurity spots had eluted and then 10 to 15% 2N ammonia/methanol in chloroform; 120 g silica column) to afford purified title compound (Rf?0.2 with 9:1 chloroform/2N ammonia in methanol as the eluant) as pale yellow solid (4.41 g, 77%).
References:
US2020/102324,2020,A1 Location in patent:Paragraph 1386; 1388

75726-96-4
105 suppliers
$50.00/1g

39890-42-1
44 suppliers
$45.00/50mg