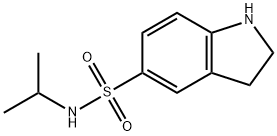
N-Isopropylindoline-5-sulfonamide synthesis
- Product Name:N-Isopropylindoline-5-sulfonamide
- CAS Number:893761-52-9
- Molecular formula:C11H16N2O2S
- Molecular Weight:240.32
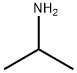
75-31-0
413 suppliers
$14.00/25mL

199328-31-9
69 suppliers
$140.00/250mg

893761-52-9
11 suppliers
$302.00/1g
Yield:893761-52-9 45%
Reaction Conditions:
with pyridine in dichloromethane at 20; for 4 h;
Steps:
5-Isopropylaminosulfonyl-2-oxindole
A suspension of 3 g 5-chlorosulfonyl-2-oxindole, 1.15 g isopropylamine and 1.2 mL of pyridine in 50 mL of dichloromethane was stirred at room temperature for 4 hours during which time a white solid formed. The solid was collected by vacuum filtration, slurry-washed with hot ethanol, cooled, collected by vacuum filtration and dried under vacuum at 40° C. overnight to give 1.5 g (45%) of 5-isopropylaminosulfonyl-2-oxindole. 1H NMR (360 MHz, DMSO-d6) δ 10.69 (s, br, 1H, NH), 7.63 (dd, J=2 and 8 Hz, 1H), 7.59 (d, J=2 Hz, 1H), 7.32 (d, J=7 Hz, 1H, NH-SO2-), 6.93 (d, J=8 Hz, 1H), 3.57 (s, 2H), 3.14-3.23 (m, 1H, CH-(CH3)2), 0.94 (d, J=7 Hz, 6H, 2*CH3).
References:
US6878733,2005,B1 Location in patent:Page/Page column 179
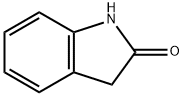
59-48-3
462 suppliers
$5.00/1g

893761-52-9
11 suppliers
$302.00/1g