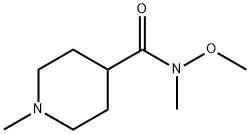
N-Methoxy-N,1-diMethylpiperidine-4-carboxaMide synthesis
- Product Name:N-Methoxy-N,1-diMethylpiperidine-4-carboxaMide
- CAS Number:215950-19-9
- Molecular formula:C9H18N2O2
- Molecular Weight:186.25
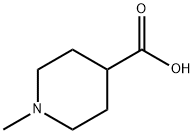
68947-43-3
169 suppliers
$10.00/1g

6638-79-5
555 suppliers
$6.00/25g

215950-19-9
44 suppliers
inquiry
Yield: 80%
Reaction Conditions:
Stage #1:1-methylpiperidine-4-carboxylic acid;N,O-dimethylhydroxylamine*hydrochloride with benzotriazol-1-ol;1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride;N-ethyl-N,N-diisopropylamine in DMF (N,N-dimethyl-formamide) for 63 h;
Stage #2: with sodium hydroxide in water
Steps:
2 Preparation 2; N-methoxy-N-methyl(1-methylisonipecotamide)
1-Methylisonipecotic acid (5.5 g, 38.4 mmol) was dissolved in dimethylformamide (100 ml) with heating. Diisopropylethylamine (8.0 ml, 46.1 mmol), 1-hydroxybenzotriazole (5.2 g, 38.4 mmol), and N,O-dimethylhydroxylamine hydrochloride (4.1 g, 42.2 mmol) were added and the reaction mixture was stirred 5 min. 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (7.4 g, 38.4 mmol) was added and the resulting homogeneous solution was stirred for 63 hours at ambient temperature. The solvent was removed under reduced pressure. The residue was dissolved in water and the solution was basified to pH 9 with 5N sodium hydroxide solution. This aqueous solution was extracted with methylene chloride then saturated with sodium chloride and extracted with chloroform/isopropanol (3/1). The combined organic extracts were dried over sodium sulfate and the solvent was removed under reduced pressure to give 9.5 g of a yellow liquid. Purification by flash chromatography (silica gel, methylene chloride:methanol:ammonium hydroxide, 100:10:1) gave 5.7 g (80%) of product as a light yellow liquid. [00381] MS (m/e): 186(M+). [00382] Analysis for C9H18N2O2:
References:
Eli Lilly and Company US6777428, 2004, B1 Location in patent:Page column 24-25