
N-Methoxy-N,2-dimethyl-oxazole-4-carboxamide synthesis
- Product Name:N-Methoxy-N,2-dimethyl-oxazole-4-carboxamide
- CAS Number:875553-59-6
- Molecular formula:C7H10N2O3
- Molecular Weight:170.17
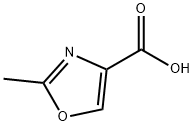
23012-17-1
87 suppliers
$15.00/250mg

6638-79-5
554 suppliers
$6.00/25g

875553-59-6
14 suppliers
$91.00/1g
Yield:875553-59-6 89.58%
Reaction Conditions:
Stage #1: 2-methyl-1,3-oxazol-4-carboxylic acidwith benzotriazol-1-ol;1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride;triethylamine in dichloromethane at 0; for 0.5 h;
Stage #2: N,O-dimethylhydroxylamine*hydrochloride in dichloromethane at 20; for 18 h;
Steps:
81.1 N-methoxy-N,2-dimethyloxazole-4-carboxamide 81b
To a solution of 2-methyloxazole-4-carboxylic acid 81a (500 mg, 3.94 mmol) in DCM (15 mL) was added TEA (1.9 mL, 13.79 mmol), the solution was cooled to 0°C. Then HOBt (585 mg, 4.33 mmol) and EDCI (906 mg, 4.73 mmol) were added. The reaction mixture was stirred at 0°C for 30min. N,O- D i methylhydroxyl amine hydrochloride (460 mg, 4.73 mmol) was added. The reaction was stirred at r.t for 18h. Water (50 mL) was added and the mixture was extracted with DCM (50 mL x 2). The combined organic layers were washed with brine, dried over anhydrous Na2SC>4 and concentrated. The residue was purified by silica gel chromatography (eluting with hexane/EtOAc = 1/1) to afford the tittle compound 81b (600 mg, 3.53 mmol, 89.58% yield).XH NMR (400 MHz, CDCb): d 8.07 (s, 1H), 3.74 (s, 3H), 3.37 (s, 3H), 2.51 (s, 3H).
References:
WO2021/158626,2021,A1 Location in patent:Page/Page column 173-174
10200-43-8
70 suppliers
$16.00/100mg

875553-59-6
14 suppliers
$91.00/1g