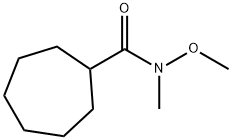
N-Methoxy-N-MethylcycloheptanecarboxaMide synthesis
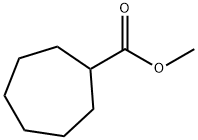
- Product Name:N-Methoxy-N-MethylcycloheptanecarboxaMide
- CAS Number:253429-08-2
- Molecular formula:C10H19NO2
- Molecular Weight:185.26
Yield:253429-08-2 84.44%
Reaction Conditions:
with O-(1H-benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate;triethylamine in N,N-dimethyl-formamide at 25; for 16 h;
Steps:
72A N-methoxy-N-methyl-cycloheptanecarboxamide
Triethylamine (10.67 g, 105.48 mmol, 14.62 mL), N-methoxymethylamine (3.60 g, 36.92 mmol) and HBTU (14.67 g, 38.68 mmol) were added into a solution of cycloheptylcarboxylic acid (5.00 g, 35.16 mmol) in DMF (50 mL) at 25°C, and the mixture was stirred for 16 h.
The reaction solution was diluted with water (200 mL), and extracted with ethyl acetate (50 mL * 6).
The organic phase was combined, washed with brine (100 mL), dried over anhydrous sodium sulfate, filtered and concentrated.
The residue was purified by column chromatography to give the title compound (colorless oil, 5.50 g, yield of 84.44%).
1H NMR (400MHz, CHLOROFORM-d) δ = 3.71 (d, J=1.5 Hz, 3H), 3.19 (s, 3H), 2.85 (br s, 1H), 1.88 - 1.61 (m, 9H), 1.49 (br s, 3H).
References:
EP3418282,2018,A1 Location in patent:Paragraph 0613-0614
6557-86-4
23 suppliers
$134.31/250mgs:

6638-79-5
555 suppliers
$6.00/25g

253429-08-2
15 suppliers
inquiry
1460-16-8
136 suppliers
$25.00/1g

6638-79-5
555 suppliers
$6.00/25g

253429-08-2
15 suppliers
inquiry