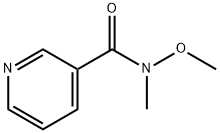
N-Methoxy-N-methylnicotinamide synthesis
- Product Name:N-Methoxy-N-methylnicotinamide
- CAS Number:95091-91-1
- Molecular formula:C8H10N2O2
- Molecular Weight:166.18

6638-79-5
559 suppliers
$6.00/25g
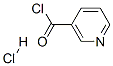
20260-53-1
201 suppliers
$16.00/5g

95091-91-1
43 suppliers
$60.00/50mg
Yield: 74%
Reaction Conditions:
with pyridine in dichloromethane at 0 - 20; for 4.25 h;
Steps:
N-Methoxy-N-methyl-nicotinamide. (compound 2, Scheme 1 ); Nicotinoyl chloride hydrochloride (compound 1 ) (10 g, 56 mmol) and 6.28 g of N, O- dimethyl-hydroxylamine.HCI (72.8 mmol) were combined in 200 ml dichloromethane. To this mixture was added 18.14 ml of pyridine (in 15 minutes at O0C). The reaction mixture was subsequently stirred for 4 hours at room temperature. The reaction was concentrated in vacuo. The resulting residue was taken up in dichloromethane and H2O (O0C), washed with a 2N NaOH solution followed by brine, dried (Na2SO4), filtered and concentrated in vacuo. Purification by flash chromatography (MeOH/triethylamine 97/3) afforded compound 2 as an oil (6.92 g, 74%). 1H- NMR (200 MHz, CDCI3) δ 8.96 (d, J = 2 Hz, 1 H), 8.69 (d, J = 5 Hz, 2 Hz, 1 H), 8.04 (dt, J = 8 Hz, 2 Hz, 1 H), 7.41 -7.32 (m, 1 H), 3.56 (s, 3H), 3.40 (s, 3H). (TLC MeOH/triethylamine Rf 0.19).
References:
SOLVAY PHARMACEUTICALS B.V. WO2008/129054, 2008, A2 Location in patent:Page/Page column 28

59-67-6
1104 suppliers
$7.00/25g

6638-79-5
559 suppliers
$6.00/25g

95091-91-1
43 suppliers
$60.00/50mg
10400-19-8
54 suppliers
inquiry

6638-79-5
559 suppliers
$6.00/25g

95091-91-1
43 suppliers
$60.00/50mg

59-67-6
1104 suppliers
$7.00/25g

95091-91-1
43 suppliers
$60.00/50mg
626-55-1
539 suppliers
$5.00/5 / G
201230-82-2
1 suppliers
inquiry

6638-79-5
559 suppliers
$6.00/25g

95091-91-1
43 suppliers
$60.00/50mg