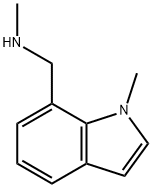
N-methyl-(1-methyl-1H-indol-7-yl)methylamine synthesis
- Product Name:N-methyl-(1-methyl-1H-indol-7-yl)methylamine
- CAS Number:709649-75-2
- Molecular formula:C11H14N2
- Molecular Weight:174.24
![Methanamine, N-[(1-methyl-1H-indol-7-yl)methylene]-](/CAS/20210305/GIF/163729-92-8.gif)
163729-92-8
0 suppliers
inquiry

709649-75-2
15 suppliers
$137.00/250mg
Yield:-
Reaction Conditions:
with sodium tetrahydroborate in methanol;
Steps:
44 Preparation 44; Preparation of METHYL- (L-METHYLINDOL-7-YLMETHYL) AMINE
To a solution of indole-7-carboxaldehyde (500 mg, 3.45 mmol) in DMF (8 ML) was added sodium hydride (152 mg of 60% dispersion in oil, 3.8 mmol). The mixture was stirred for 30 mins. Methyl iodide (0.98 g, 6.9 mmol) was then added and the mixture was stirred for 2 hrs. Ethyl acetate (200 mL) was added and solution was washed with H20 (3 x 20 mL) and brine (25 mL) dried over MGS04 and concentrated to afford N-methylindole-7- carboxaldehyde as a brown oil which was used without further purification. The crude oil was dissolved in anhydrous methanol (10 mL). Methylamine (5.1 mL OF 2M solution in methanol, 9.55 mmol) was added and the mixture was stirred for 3 hours. The solution was concentrated to a yellow oil and then dissolved into anhydrous methanol (10 mL). Sodium borohydride (131 mg, 3.45 mmol) was added and the mixture was stirred overnight. Water (1 mL) was added and the solution was concentrated to an orange oil. Sodium hydroxide (5 mL, 1N) was added and the product was extracted with ethyl acetate (3 x 20 mL), dried over MGS04 and concentrated to afford the title compound as a brown oil (400 mg, 68%). IH NMR (200 MHz, CDC13) 8 7.52 (dd, J= 7.0, 2.0 Hz, 1H), 7.23-6. 94 (m, 3H), 6.44 (d, J= 3.1 Hz, 1H), 4.10 (s, 3H), 4.04 (s, 2H), 2.51 (s, 3H).
References:
WO2004/52890,2004,A1 Location in patent:Page 92-93