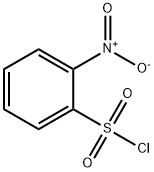
N-Methyl-2-nitrobenzenesulphonamide synthesis
- Product Name:N-Methyl-2-nitrobenzenesulphonamide
- CAS Number:23530-40-7
- Molecular formula:C7H8N2O4S
- Molecular Weight:216.21
Yield:23530-40-7 95%
Reaction Conditions:
with sodium hydroxide in water;ethyl acetate at 0 - 20; for 0.916667 h;
Steps:
N-Methyl-2-nitrobenzenesulfonamide (6)
To a cooled (0 °C) solution of methylamine hydrochloride (15.8 g, 71.3mmol) in H2O (43 mL) was added NaOH (17.2 g, 428 mmol). A solutionof 2-nitrobenzenesulfonyl chloride in EtOAc (140 mL) was addeddropwise via separatory funnel over a period of 30 min. The resultingbiphasic mixture was stirred at 0 °C for 5 additional min and was thenstirred for 20 min at r.t. The layers were then separated and the aqueouslayer was extracted with EtOAc (2 × 100 mL). The combined organiclayers were dried, filtered, and concentrated in vacuo. The residualoff-white solid was crystallized from CH2Cl2/hexanes to affordthe desired product 6 as off-white crystals; yield: 14.6 g (95%).IR (thin film): 3335, 3095, 1540 cm-1.1H NMR (CDCl3, 400 MHz): δ = 8.13 (m, 1 H), 7.86 (m, 1 H), 7.78-7.73(m, 2 H), 5.24 (br s, 1 H), 2.78 (d, J = 4.8 Hz, 3 H).1H NMR was in accordance with literature values.2113C NMR (CDCl3, 100 MHz): δ = 148.2, 133.7, 132.7, 132.4, 131.5 125.4,29.8.LC/MS (ESI): m/z [M - NH(CH3)]+ calcd for C6H5NO4S: 187.1; found:187.1.
References:
Brockway, Anthony J.;Cosner, Casey C.;Volkov, Oleg A.;Phillips, Margaret A.;De Brabander, Jef K. [Synthesis,2016,vol. 48,# 13,art. no. SS-2016-M0105-OP,p. 2065 - 2068]
![2-Cyclopentene-1-carboxamide, 4-(6-chloro-9H-purin-9-yl)-N-[(2-nitrophenyl)sulfonyl]-, (1S,4R)-](/CAS/20210305/GIF/1026978-85-7.gif)
1026978-85-7
0 suppliers
inquiry

23530-40-7
63 suppliers
inquiry