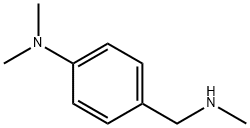
N-methyl-4-(N,N-dimethylamino)benzylamine synthesis
- Product Name:N-methyl-4-(N,N-dimethylamino)benzylamine
- CAS Number:83671-43-6
- Molecular formula:C10H16N2
- Molecular Weight:164.25
100-10-7
604 suppliers
$5.00/1g

74-89-5
7 suppliers
$13.44/25ML

83671-43-6
35 suppliers
$45.00/100mg
Yield:83671-43-6 73%
Reaction Conditions:
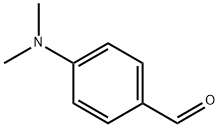
Stage #1: 4-dimethylamino-benzaldehyde;methylamine in water;acetonitrile at 0; for 2 h;Inert atmosphere;
Stage #2: with sodium cyanoborohydride in water;acetonitrile at 20; for 4 h;
Steps:
(A-N, Λ/-Dimethylaminobenzyl)-methylamine (32)To a stirred solution of 4-Λ/, Λ/-dimethylaminobenzaldehyde (3.00 g, 18.3 mmol) (29) in acetonitrile (400 ml) under N2 at 0 0C was added methylamine solution in water (40% v/v) (10.0 ml, 103 mmol) and the reaction allowed to progress for 2 hours. Then NaCNBH3 (3.46 g, 64.1 mmol) was added and the reaction allowed to progress at room temperature for 4 hours. The solvent was removed on the rotorevaporator with water removed by azeotroping with toluene. The crude product was purified directly by column chromatography using EtOAc (100%) followed by EtOAc: MeOH: NEt3 (92: 5: 3) as eluent to give 32 as a yellow oil (2.20 g, 73%); 1H NMR (CDCI3, 300 MHz) δ 7.17 (2H, d, J = 8.7 Hz), 6.70 (2H, d, J = 8.7 Hz), 3.59 (2H, s), 2.88 (6H, s), 2.34 (3H, s); 13C NMR (CDCI3, 100 MHz) δ 150.0 (2CIV)), 129.1 (2C), 112.7 (2C), 55.6, 40.3, 35.6 (2C); HRMS (ESI): Found 165.1392 (M+): C10H17N2 (M+) requires 165.1390.
References:
WO2010/18555,2010,A2 Location in patent:Page/Page column 19; 29-30
![Benzenamine, N,N-dimethyl-4-[(methylimino)methyl]-](/CAS/20180601/GIF/877-79-2.gif)
877-79-2
0 suppliers
inquiry

83671-43-6
35 suppliers
$45.00/100mg

593-51-1
532 suppliers
$21.00/100g

100-10-7
604 suppliers
$5.00/1g

83671-43-6
35 suppliers
$45.00/100mg