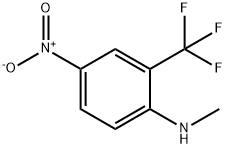
N-Methyl-4-nitro-2-(trifluoroMethyl)aniline synthesis
- Product Name:N-Methyl-4-nitro-2-(trifluoroMethyl)aniline
- CAS Number:54672-10-5
- Molecular formula:C8H7F3N2O2
- Molecular Weight:220.15
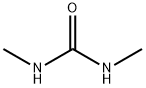
96-31-1
421 suppliers
$5.00/25g
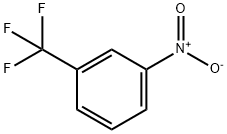
98-46-4
252 suppliers
$19.00/5g

54672-10-5
14 suppliers
$55.00/250mg
Yield:54672-10-5 95%
Reaction Conditions:
with sodium hydroxide in dimethyl sulfoxide;
Steps:
1 N-methyl-4-nitro-2-trifluoromethylaniline
Example 1 N-methyl-4-nitro-2-trifluoromethylaniline 1.91 g (10 mmol) of 3-nitrobenzotrifluoride and 1.76 g (20 mmol) of N,N'-dimethylurea are heated to 50° C. for 4 h with 1.2 g (30 mmol) of sodium hydroxide in the form of microbeads in 30 ml of DMSO, passing through an airstream. The mixture is then diluted with ethyl acetate and washed repeatedly by shaking with sainted soda solution. After drying the organic phase over sodium sulphate and taking off the solvent, 2.2 g (10 mmol, 100%) of N-methyl-4-nitro-2-trifluoromethylaniline are obtained as a 95% pure product of m.p. 99°-101° C. After recrystallization from ethanol the product has a m.p. of 111°-112° C.
References:
US5684203,1997,A
777-37-7
292 suppliers
$10.00/5g

74-89-5
7 suppliers
$13.44/25ML

54672-10-5
14 suppliers
$55.00/250mg