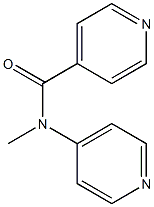
N-methyl-N-pyridin-4-ylpyridine-4-carboxamide synthesis
- Product Name:N-methyl-N-pyridin-4-ylpyridine-4-carboxamide
- CAS Number:1617537-44-6
- Molecular formula:C12H11N3O
- Molecular Weight:213.2352
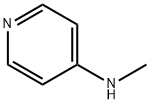
1121-58-0
180 suppliers
$20.00/1g
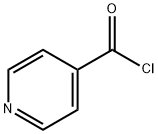
14254-57-0
18 suppliers
$1681.00/10g

1617537-44-6
6 suppliers
inquiry
Yield:1617537-44-6 90%
Reaction Conditions:
with triethylamine in dichloromethane at 20; for 24 h;
Steps:
N-Methyl-N-(4-pyridyl)pyridine-4-carboxamide, 2
To a solution of N-methyl-4-pyridinamine (334 mg, 3.09 mmol) and isonicotinoyl chloride (550 mg, 3.09 mmol) dissolved in dichloromethane (31 ml), was added triethylamine (1.08 ml, 7.73 mmol) in one portion and the reaction was left stirring overnight at room temperature, until deemed complete by TLC. After 24 hours, NaOH (1M, 30 ml) was added and the layers mixed. The organic layer was further washed with NaOH (1M, 30 ml). The combined aqueous layers were extracted with dichloromethane (30 ml) and the combined organic layers washed with water (30 ml), dried (MgSO4) and the solvent removed in vacuo. The product was purified by flash column chromatography (SiO2; 100% dichloromethane 5% methanol in dichloromethane) to yield the title compound as a colourless solid (750 mg, 90%).
References:
Fahs, Sara;Rowther, Farjana B.;Dennison, Sarah R.;Patil-Sen, Yogita;Warr, Tracy;Snape, Timothy J. [Bioorganic and Medicinal Chemistry Letters,2014,vol. 24,# 15,p. 3430 - 3433] Location in patent:supporting information