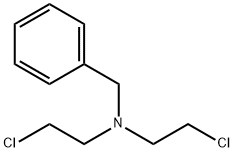
N,N-BIS(2-CHLOROETHYL)BENZENEMETHANAMINE synthesis
- Product Name:N,N-BIS(2-CHLOROETHYL)BENZENEMETHANAMINE
- CAS Number:55-51-6
- Molecular formula:C11H15Cl2N
- Molecular Weight:232.15
Yield: 81%
Reaction Conditions:
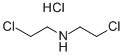
Stage #1:N-(tert-butyloxycarbonyl)bis(2-chloroethyl)amine with hydrogenchloride in 1,4-dioxane at 20; for 1 h;
Stage #2:benzaldehyde with sodium tris(acetoxy)borohydride;triethylamine in 1,2-dichloro-ethane at 20; for 54 h;
Steps:
3.a Step a:
The solution of the compound tert-butyl bis(2-chloroethyl)carbamate (11.00g,45.43mmol) in HC1/Dixoane (4mol/L, 200mL) was stirred for lh at 20°C. The solution was concentrated and the residue was dissolved in DCE (200mL). Triethylamine (22.95g,227.l4mmol) and benzaldehyde (7.23g, 68.l4mmol) was added to the mixture. And then NaBH(OAc)3 (24.07g, 113.S7mmol) was added in portionwise. The mixture was stirred for 54 hours at 20°C, and then EA(300mL) and brine (200mL) was added. The organic layer was concentrated under reduced pressure in vacuo. The residue was dissolved in HC1 solution (2mol/L, 200mL) and extracted with EA (lOOmL). The pH value of the aqueous layer was adjusted to 9 withsaturated Na2CO3 solution. The mixture was extracted with EA (200mL). The organic layer was dried over anhydrous Na2504 and concentrated to afford the compound
References:
JACOBIO PHARMACEUTICALS CO., LTD.;JACOBIO-ALPHA PHARMACEUTICALS CO., LTD.;MA, Cunbo;GAO, Panliang;HU, Shaojing;XU, Zilong;HAN, Huifeng;WU, Xinping;KANG, Di;LONG, Wei WO2018/172984, 2018, A1 Location in patent:Page/Page column 84; 85

101-32-6
138 suppliers
$31.00/1g

55-51-6
58 suppliers
inquiry

10429-82-0
158 suppliers
$7.00/1g

55-51-6
58 suppliers
inquiry